
As filed with the Securities and Exchange Commission on July 31, 2007
Registration No. 333-143160
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
AMENDMENT NO. 4
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AMEDICA CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 3841 | 84-1375299 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(IRS Employer Identification No.) |
615 Arapeen Drive
Suite 302
Salt Lake City, Utah 84108
(801) 583-5100
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Ashok C. Khandkar, Ph.D.
Chief Executive Officer
Amedica Corporation
615 Arapeen Drive
Suite 302
Salt Lake City, Utah 84108
(801) 583-5100
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
With copies to:
| Jonathan L. Kravetz, Esq. Anthony E. Hubbard, Esq. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. One Financial Center Boston, MA 02111 (617) 542-6000 |
Bruce K. Dallas, Esq. Davis Polk & Wardwell 1600 El Camino Real Menlo Park, CA 94025 (650) 752-2000 |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are being offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. ¨
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), shall determine.
The Information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell securities, and we are not soliciting offers to buy these securities, in any state where the offer or sale is not permitted.
PROSPECTUS (Subject to Completion)
Issued July 31, 2007
4,650,000 Shares

COMMON STOCK
Amedica Corporation is offering 4,650,000 shares of its common stock. This is our initial public offering and no public market currently exists for our shares. We anticipate that the initial public offering price will be between $13 and $15 per share.
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “AMCA.”
Investing in our common stock involves risks. See “ Risk Factors” beginning on page 8.
PRICE $ A SHARE
| Price to Public |
Underwriting Discounts and Commissions |
Proceeds to Amedica | ||||
| Per Share |
$ | $ | $ | |||
| Total |
$ | $ | $ |
We have granted the underwriters the right to purchase up to an additional 697,500 shares of common stock to cover over-allotments.
The Securities and Exchange Commission and state securities regulators have not approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares to purchasers on or about , 2007.
| MORGAN STANLEY |
| JEFFERIES & COMPANY |
| CIBC WORLD MARKETS |
, 2007
You should rely only on the information contained in this prospectus or contained in any free writing prospectus that we may authorize to be delivered to you. We have not, and the underwriters have not, authorized any other person to provide you with information different from, or in addition to, that contained in this prospectus or any related free writing prospectus. We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus or any related free writing prospectus is accurate only as of its date, regardless of the time of its delivery, or of any sale of common stock.
Through and including , 2007 (25 days after the date of this prospectus), all dealers that buy, sell or trade shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
This summary highlights what we believe are the most important features of this offering and the information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before investing in our common stock. You should read the entire prospectus carefully, including “Risk Factors” and our financial statements and the related notes included in this prospectus. Unless the context requires otherwise, references to “Amedica,” “we,” “our” and “us” in this prospectus refer to Amedica Corporation.
AMEDICA CORPORATION
Overview
We are an orthopedic implants company focused on using our silicon nitride ceramic technologies to develop, manufacture and commercialize a broad range of advanced, high-performance spine and joint implants. We have developed a formulation of silicon nitride which we believe has the strength, toughness and wear resistance necessary to overcome the limitations of currently available orthopedic implants. Upon introduction to market, we believe our implants will represent the first commercial use of silicon nitride ceramics in orthopedic applications and will have the potential to provide an improved combination of characteristics, including greater strength and resistance to fracture, improved resistance to wear, greater ability to promote bone attachment and better compatibility with surgical and diagnostic imaging. Based on these potential advantages, we believe our silicon nitride product candidates may achieve better long-term clinical outcomes with enhanced durability, longevity, biocompatibility and patient fit. While we have not received regulatory clearance or approval for any of our product candidates that we intend to commercialize, in the second half of 2007 we expect to submit premarket notifications to the U.S. Food and Drug Administration, or the FDA, seeking regulatory clearance of our first commercial product candidate. Our goal is to establish our silicon nitride implants as new standards of care for the largest and fastest growing orthopedic implant markets: the spine, hip and knee markets.
Our lead product candidates are our Valeo™ family of spinal implants, which are intended to restore and maintain the alignment of vertebrae in the cervical, or neck, region and lumbar, or lower back, region of the spine. We expect to launch the first of these products by mid-2008, subject to clearance by the FDA. In 2006, we received clearance from the FDA for a silicon nitride ceramic spinal spacer, a device for insertion between two vertebrae to help stabilize the spine. We believe this is the first ceramic spinal spacer ever cleared by the FDA for human use. Although we do not plan to commercialize this spinal spacer, it will be the predicate device for our Valeo spinal spacers. We plan to introduce additional spinal spacers by the end of 2008, subject to regulatory clearance, including cortico-cancellous spacers that feature a bone-like structure with a solid, or cortical, load-bearing portion and a cancellous, or porous, structure that is intended to promote bone attachment for spinal fixation. In mid-2009, subject to regulatory approval, we plan to introduce cortico-cancellous spinal spacers with a surface coating designed to enhance bone attachment. Our Valeo family of spinal implant candidates also includes an all-ceramic, motion-preserving cervical disc, for which we anticipate commencing a clinical trial by mid-2009.
In addition, we are incorporating our silicon nitride ceramic technology into the development of our Infinia™ family of total hip and knee implants. We anticipate performing clinical trials for each of our Infinia product candidates. We believe that our Infinia Total Hip and Knee Implants, if approved or cleared by the FDA, may provide competitive advantages over currently marketed total hip and knee replacement implants. We are designing our Infinia Total Hip Implant to offer surgeons a range of sizes and design options comparable to implants with metal and plastic components but with improved wear resistance. We anticipate commencing a clinical trial for the first of these total hip implant candidates in 2009. We also are designing our Infinia Total Knee Implant and anticipate commencing a clinical trial for this product candidate in 2010. We believe that its
1
design will provide natural anatomic motion and offer lower wear and improved longevity compared to currently marketed knee replacement implants.
During the past two years, we have been designing and constructing our own manufacturing facility and developing processes that will provide us the ability to control the commercial-scale production of our silicon nitride ceramic implants from powder form to devices ready for sterilization and packaging. We are currently producing our lead ceramic spinal product candidates on a pilot scale in our manufacturing facility. We anticipate our facility will be fully operational for commercial-scale production by the end of 2007, which we believe would make us the only vertically integrated silicon nitride orthopedic implant manufacturer in the world.
Our Market Opportunity
According to the Millennium Research Group, in 2006, patients in the United States had approximately 1.5 million spinal fixation, hip replacement and knee replacement surgical procedures performed involving the use of implants, and this number is expected to grow primarily due to the rising incidence of arthritis. In 2005, an estimated 46 million U.S. adults suffered from doctor-diagnosed arthritis, and nearly two-thirds of those afflicted were younger than age 65. Osteo-arthritis, a condition involving the degeneration, or wearing away, of the cartilage at the end of bones, is a common form of arthritis, and often results in progressive joint disease and pain. The prescribed treatment for osteo-arthritis disorders depends on the severity and duration of the disorder and ranges from non-operative procedures including bed rest, medication, lifestyle modifications, exercise, physical therapy, chiropractic care and steroid injections, to surgical intervention including total joint replacement. In cases where surgical intervention is prescribed, the use of implants has evolved into the standard of care in spine, hip and knee surgery.
The spine market is the fastest growing market for orthopedic implants, accounting for $3.3 billion in sales in the United States in 2006, and is projected to grow at an average annual rate of 12.0% through 2011 to approximately $5.9 billion. Spinal fixation surgeries currently represent the vast majority of procedures in this market. Approximately 500,000 spinal fixation surgery procedures were performed in the United States in 2006, accounting for approximately $3.2 billion of the total $3.3 billion in U.S. spine implant market.
Orthopedic implants used in hip and knee replacement surgeries generated approximately $5.6 billion in sales in 2006 in the United States, and such sales are projected to increase at an average annual rate of 9.4% through 2011 to approximately $8.8 billion. Approximately one million primary hip and knee replacement procedures were performed in the United States in 2006.
We believe that the market for implants used in spine, hip and knee surgical procedures will continue to grow because of the following market dynamics:
| • | growth of the aging population; |
| • | changing lifestyle expectations; |
| • | earlier surgical intervention; |
| • | rising number of revision surgeries; |
| • | introduction of new technologies; and |
| • | market expansion into new geographic areas. |
2
Our Solution
We believe our silicon nitride ceramic technologies, MC2 and CSC, will overcome many of the limitations associated with currently available implant materials by providing an improved combination of characteristics, including:
| • | greater strength and resistance to fracture than currently marketed ceramic implants; |
| • | improved resistance to wear compared to implants made of plastics and metals; |
| • | greater ability to promote bone attachment than traditional plastic and metal implants such as polyetheretherketone, or PEEK, and titanium; and |
| • | better compatibility with surgical and diagnostic imaging techniques. |
We believe that the anticipated greater strength and fracture resistance of our silicon nitride implant candidates will allow us to offer a wider range of design and size options and lower risk of fracture compared to currently marketed implants made of ceramic materials. We further believe that the anticipated improved wear resistance and biocompatibility over the life of our silicon nitride product candidates will reduce the risk of bone loss and allergic response to metal wear particles. Based on these potential advantages, we believe that our silicon nitride product candidates will achieve better long-term clinical outcomes with a combination of improved durability, longevity, biocompatibility and patient fit. Our ceramic product categories include:
| • |
Micro-Composite Ceramic, or MC2. We refer to our formulation of silicon nitride as MC2, or Micro-Composite Ceramic. We expect that all of our ceramic product candidates will be made using our MC2 silicon nitride. |
| • |
Cortico-cancellous Structured Ceramics, or CSC. We also are developing silicon nitride ceramic implants that mimic the structure of natural bone by incorporating both a dense load-bearing component and a porous component, coupled with a surface coating, intended to promote bone attachment. We call our ceramic implants based on this technology CSC, or Cortico-cancellous Structured Ceramic, implants. |
Our Strategy
Our goal is to become a leading orthopedic company offering advanced silicon nitride ceramic implants for a broad range of orthopedic indications. We intend to use our ceramic technologies to develop implants that have performance advantages compared to existing implants. We believe that the combined benefits of our MC2 and CSC technologies will give our product candidates the potential to become a new standard of care for spine, hip and knee procedures.
Key elements of our strategy to achieve this objective include the following:
| • | launch near-term product candidates that address substantial market opportunities and build market awareness; |
| • | build a broad portfolio of ceramic implants targeting expanded indications and additional surgical procedures; |
| • | utilize the expertise of our surgeon advisors to design physician-preferred product features; |
| • | establish a hybrid sales organization utilizing experienced, independent sales agencies and a direct sales force; and |
| • | selectively establish collaborations for our implants with leading orthopedic companies. |
3
Our Product Candidates
We are using our MC2 and CSC ceramic technologies to develop and commercialize innovative orthopedic implant products for the spine, hip and knee implant markets.
Our Spinal Implant Products
We have designed our lead product candidates in our Valeo family of spinal implants as a comprehensive solution for surgical procedures for spinal fixation. These products, if cleared or approved by the FDA, include spinal spacers, a cervical bone plate system, a pedicle screw system, and a set of surgical instruments that facilitate the placement of our implants in the body. We are also developing an all-ceramic motion-preserving cervical disc.
Valeo Cortical, Cortico-Cancellous, and Coated Cortico-Cancellous Spinal Spacers. We have designed our Valeo family of spinal spacers, using silicon nitride ceramic, as intervertebral fixation implants for stabilizing the spine that replace a portion of a vertebra that has collapsed, been damaged, or becomes unstable due to disease or trauma. We believe that each of our Valeo Spinal Spacers will have competitive advantages compared to existing spinal implants. We developed and received FDA clearance for our ArxTM Intervertebral Spacers made from MC2 silicon nitride, which will serve as the predicate device for the 510(k) premarket notification for our Valeo Cortical Spinal Spacers product candidate.
Valeo Cervical Plate System and Pedicle Screw System. We are developing our Valeo Cervical Plate System and Valeo Pedicle Screw System as titanium alloy supplemental fixation implants to be used in conjunction with our Valeo Spinal Spacers. Our design and instruments combine special features to enable surgeons, in a single step, to hold the cervical plate in place, ensure proper angling and insertion of the screws into the vertebrae, and achieve a consistent supplemental fixation outcome. Our screw system incorporates modularity in the system components to permit such flexibility, which we believe will provide better clinical outcomes.
Valeo Cervical Disc. We are developing our Valeo Cervical Disc, using both our MC2 and CSC technologies, as a silicon nitride ceramic implant to meet the unmet market need for a disc replacement implant that will restore natural motion and provide uncompromised wear resistance and favorable imaging characteristics in the cervical spine. We believe our Valeo Cervical Disc will represent a significant advance over currently available disc implants.
Our Hip Implant Products
Infinia Total Hip Implant. We are developing our Infinia Total Hip Implant for patients undergoing total hip replacement surgery for the treatment of degenerative joint disease. In our first hip replacement implant, we will use silicon nitride ceramic for the femoral head component of this implant. The counter-bearing, or mating component, of the hip implant, will be a polyethylene liner, fixed into a metal acetabular cup, using industry-recognized designs and materials. We anticipate that our Infinia Total Hip Implant, if cleared by the FDA based on a 510(k) premarket notification supported by sufficient clinical trial results, will provide competitive advantages over traditional total hip replacement implants presently on the market.
Infinia Total Hip Implant II. We are developing our second generation Infinia Total Hip Implant II which, if approved by the FDA, will feature our Infinia monoblock cup, an industry-first, one-piece, fully ceramic acetabular cup, our large diameter Infinia ceramic and metal femoral heads, and our Infinia femoral stem. The Infinia monoblock cup will be made from silicon nitride ceramic and will incorporate a smooth bearing surface on the inside of the cup integrated with a bone attachment surface incorporating our CSC technology on the outside of the cup that comes into contact with a patient’s pelvis. The femoral head of the implant will be a large-diameter head offered in two versions, one made of silicon nitride and the other of cobalt-chromium metal alloy.
4
The femoral head will be used with a metal stem inserted into the femur. In contrast to currently marketed ceramic femoral heads, we are designing our MC2 femoral head to offer surgeons a range of size and design options comparable to those available in metal femoral heads.
Our Knee Implant Product
Infinia Total Knee Implant. Our Infinia Total Knee Implant will incorporate silicon nitride bearing components for the femoral condyle. The tibial tray will be made from traditional metal. The tibial insert will be made from polyethylene in a rotating platform design intended to give the knee implant a range of motion and flexion similar to the natural knee. We anticipate that this total knee replacement product candidate, if approved by the FDA, will provide natural anatomic motion and will offer a low-wear knee replacement option, providing significantly improved longevity compared with current metal-on-polyethylene knee implants.
Risks Associated with Our Business
Our business is subject to a number of risks that you should be aware of before making an investment decision. These risks are discussed more fully in the section of this prospectus entitled “Risk Factors.” We have not received regulatory clearance or approval to commercialize our Valeo or Infinia product candidates for any intended use. If we are unable to successfully develop, receive regulatory clearance or approval for and commercialize our implant products, we may never generate revenue or be profitable and may have to cease operations. We have a limited operating history and no products in commercial distribution. To date, our only significant revenue has been from research grants from the National Institutes of Health and with the exception of a small net income for the years ended December 31, 2002 and 1999, we have incurred net losses in each year since our inception. Our ability to expand the use of our ceramic technologies may be limited by a number of factors, including intellectual property held by other parties. Our competitors and potential competitors include much larger companies with more resources and commercialization experience than we have. We have generated no revenues from operations, and as of March 31, 2007, we had an accumulated deficit during the development stage of $18.2 million. We expect to continue to incur additional, and possibly increasing, losses through at least the end of 2010.
Corporate Information
We were incorporated in Delaware in 1996 under the name Amedica Corp. and have since changed our name to Amedica Corporation. Our principal executive offices are located at 615 Arapeen Drive, Suite 302, Salt Lake City, Utah 84108, and our telephone number is (801) 583-5100. Our web site address is www.amedicacorp.com. The information on, or that may be accessed through, our web site is not incorporated by reference into this prospectus and should not be considered a part of this prospectus. As used in this prospectus, references to “we,” “our,” “us” and “Amedica” refer to Amedica Corporation unless the context requires otherwise.
Certain monetary amounts, percentages and other figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables may not be the arithmetic aggregation of the figures that precede them, and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
We have applied for federal registration of the marks “Altia”, “AMCA”, “Amedica”, “CSC”, “Improving Function. Enhancing Lives.”, “Infinia”, “Infinite Possibility”, “MC2”, and “Valeo”. All other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
5
THE OFFERING
| Common stock offered by us |
4,650,000 shares |
| Common stock to be outstanding after this offering |
15,318,818 shares |
| Over-allotment option |
697,500 shares |
| Use of proceeds |
We intend to use the net proceeds from this offering to fund the development and commercialization of our lead products, build our sales, marketing and distribution capabilities, establish commercial-scale manufacturing operations, fund research and development activities for our pipeline products and for other general corporate purposes. See “Use of Proceeds.” |
| Proposed NASDAQ Global Market symbol |
AMCA |
The information above is based on 2,329,522 shares of common stock outstanding as of June 30, 2007, and assumes the conversion of all of our preferred stock outstanding as of June 30, 2007, into 8,339,296 shares of common stock upon the completion of this offering. It does not include:
| • | 972,888 shares of common stock issuable upon the exercise of outstanding options to purchase common stock, at a weighted average exercise price of $2.55 per share; |
| • | 1,062,067 shares of common stock issuable upon the exercise of warrants for shares of Series A, Series B, Series C and Series D convertible preferred stock, on an as-converted basis, outstanding as of June 30, 2007, at a weighted average exercise price of $5.20 per share; |
| • | 99,099 additional shares of common stock reserved for issuance under our 2003 Stock Option Plan; and |
| • | 2,000,000 additional shares of common stock reserved for issuance under our 2007 Stock Plan, which becomes effective upon completion of this offering. |
Unless otherwise indicated, all information contained in this prospectus:
| • | assumes that the underwriters do not exercise their over-allotment option; |
| • | reflects a 1-for-3.82 reverse split of our common stock effected on July 26, 2007; |
| • | reflects the automatic conversion of all of our outstanding shares of preferred stock into 8,339,296 shares of common stock upon completion of this offering; |
| • | reflects the conversion of all outstanding warrants exercisable for shares of preferred stock into warrants exercisable for shares of common stock upon completion of this offering; and |
| • | assumes the adoption of our amended and restated certificate of incorporation and amended and restated bylaws upon the completion of this offering. |
6
SUMMARY FINANCIAL DATA
The summary financial data set forth below should be read in conjunction with our financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| Years Ended December 31, | Three Months Ended March 31, |
Period
from March 31, 2007 |
||||||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||||||
| Grant revenue |
$ | 304,333 | $ | 299,583 | $ | 208,252 | $ | 69,207 | $ | 94,850 | $ | — | $ | — | $ | 1,234,476 | ||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Research and development |
207,298 | 380,771 | 1,419,293 | 2,966,991 | 4,974,380 | 1,100,125 | 1,479,340 | 11,802,694 | ||||||||||||||||||||||||
| General and administrative |
67,551 | 142,377 | 398,208 | 576,295 | 1,113,500 | 184,425 | 405,380 | 2,806,322 | ||||||||||||||||||||||||
| Sales and marketing |
— | — | — | 416,847 | 607,538 | 111,038 | 125,740 | 1,150,125 | ||||||||||||||||||||||||
| Total operating expenses |
274,849 | 523,148 | 1,817,501 | 3,960,133 | 6,695,418 | 1,395,588 | 2,010,460 | 15,759,141 | ||||||||||||||||||||||||
| Income (loss) from operations |
29,484 | (223,565 | ) | (1,609,249 | ) | (3,890,926 | ) | (6,600,568 | ) | (1,395,588 | ) | (2,010,460 | ) | (14,524,665 | ) | |||||||||||||||||
| Interest income (expense), net |
(16,705 | ) | (6,863 | ) | 107,211 | 248,838 | 727,939 | 150,487 | 129,148 | 1,153,775 | ||||||||||||||||||||||
| Change in value of preferred stock warrants |
— | — | (254,089 | ) | (577,000 | ) | (290,925 | ) | (72,731 | ) | (3,681,413 | ) | (4,803,427 | ) | ||||||||||||||||||
| Net income (loss) |
$ | 12,779 | $ | (230,428 | ) | $ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,317,832 | ) | $ | (5,562,725 | ) | $ | (18,174,317 | ) | |||||||||
| Basic and diluted net income (loss) per share |
$0.01 | $(0.11) | $(0.78) | $(1.87) | $(2.72) | $(0.58) | $(2.45) | |||||||||||||||||||||||||
| Weighted average number of shares outstanding—basic and diluted |
2,094,241 | 2,123,281 | 2,247,611 | 2,254,454 | 2,267,464 | 2,265,863 | 2,271,986 | |||||||||||||||||||||||||
| As of March 31, 2007 | ||||||||||
| (unaudited) | ||||||||||
| Actual | Pro Forma(1) | Pro Forma as Adjusted(1)(2) | ||||||||
| Balance Sheet Data: |
||||||||||
| Cash, cash equivalents and marketable securities |
$ | 10,325,322 | $ | 22,725,322 | $ | 80,518,322 | ||||
| Working capital |
|
9,541,106 |
|
21,941,106 | 79,734,106 | |||||
| Total assets |
17,207,969 | 29,607,969 | 87,400,969 | |||||||
| Long-term debt, including current portion |
|
1,556,241 |
|
|
1,556,241 |
1,556,241 | ||||
| Convertible preferred stock |
|
26,389,982 |
|
— | — | |||||
| Total stockholders’ equity (deficit) |
(17,559,966 | ) |
|
27,534,979 |
85,327,979 | |||||
| (1) | The pro forma balance sheet data above reflect our unaudited capitalization as of March 31, 2007, on a pro forma basis giving effect to (i) the issuance of 4,456,500 shares of our Series D convertible preferred stock and warrants exercisable for 253,290 shares of our Series D convertible preferred stock in April 2007, for aggregate net proceeds of approximately $12.4 million, (ii) the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 8,339,296 shares of our common stock upon the completion of this offering, and (iii) the conversion of all outstanding warrants to purchase shares of our convertible preferred stock into warrants to purchase an aggregate of 1,062,067 shares of our common stock (but not assuming the exercise of these common stock warrants) upon the completion of this offering, and the related reclassification of the preferred stock warrant liability to additional paid in capital. |
| (2) | The pro forma as adjusted balance sheet data above reflect the issuance of 4,650,000 shares of our common stock upon the completion of this offering at an assumed initial public offering price of $14.00 per share (the midpoint of the range on the front cover of this prospectus) after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
7
An investment in our common stock involves a high degree of risk. You should carefully read and consider the risks described below, as well as the other information in this prospectus, including our financial statements and the related notes, before deciding to invest in our common stock. If any of the following risks occur, our business, financial condition, results of operations or cash flows could be materially harmed. In that case, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business and Strategy
We are an early stage company with no product revenues, and if we fail to execute effectively on all elements of our business plan, we may not succeed in our goal of becoming a profitable orthopedic implants company.
We have not yet commercialized any of our product candidates. We have four spinal implant product candidates that we intend to introduce to market in the United States in 2008, and one spinal spacer candidate we plan to introduce in mid-2009, by seeking 510(k) regulatory clearance for each of them from the FDA. We refer to these implants as our lead product candidates due to the regulatory pathway we intend to pursue and because we plan to introduce each of them to market within the next two years. We refer to implants as our pipeline product candidates if we expect to conduct clinical trials in support of regulatory clearance or approval from the FDA and, therefore, we plan to introduce these product candidates to market in the United States over a longer term. We do not expect to start clinical trials of the earliest of our pipeline product candidates before the first half of 2009. There is no assurance that we will succeed in bringing any of our product candidates to market. In order to succeed in our commercialization efforts, we must execute effectively on all elements of our business plan, including product development and testing, obtaining regulatory clearances and approvals, establishing our sales and marketing capabilities, and developing certified, validated and effective commercial-scale manufacturing operations. If we fail in any of these endeavors, or experience delays in pursuing them, we will not generate revenues as planned and will need to curtail operations or seek additional financing earlier than otherwise anticipated.
Our near-term success depends substantially on our ability to obtain regulatory clearance or approval and thereafter commercialize our most advanced spinal implant product candidates; we cannot be certain that we will be able to do so in a timely fashion or at all.
The process of obtaining regulatory clearances or approvals to market a medical device from the U.S. Food and Drug Administration, or the FDA, or similar regulatory authorities outside of the United States can be costly and time consuming, and there can be no assurance that such clearances or approvals will be granted on a timely basis, or at all. The FDA’s 510(k) clearance process generally takes one to six months from submission, depending on whether a Special or traditional 510(k) premarket notification has been submitted, but can take significantly longer. An application for premarket approval, or PMA, must be submitted to the FDA if the device cannot be cleared through the 510(k) clearance process or is not exempt from premarket review by the FDA. The PMA process almost always requires one or more clinical trials and can take two to three years from the date of filing, or even longer. In some cases, including in the case of our Inifinia Total Hip Implant, the FDA has indicated that it will require clinical data as part of the 510(k) process.
Our regulatory strategy is to try to accelerate market introduction of our most advanced product candidates by submitting either a traditional or a Special 510(k). We currently intend to seek Special 510(k) clearance for certain of our lead spinal implant products under development. We expect to submit a 510(k) for our Infinia Total Hip Implant, which will include our ceramic femoral head, and we anticipate that the FDA will require clinical trials in support of this 510(k) as well as for our applications through the PMA process for the rest of our pipeline product candidates.
There is no certainty, however, that any of our lead product candidates, particularly those incorporating silicon nitride ceramic materials, will be cleared by the FDA by means of either a traditional or a Special 510(k).
8
In correspondence relating to a 510(k) we submitted for spinal product candidates we previously were developing using zirconia-toughened alumina, the FDA raised a number of questions regarding the strength of the zirconia-toughened alumina ceramic under biological conditions, the binding of hydroxy-apatite to the ceramic, the ability of the devices to facilitate adequate bone attachment and the implications of potential wear debris generated during implantation of the devices. We withdrew our 510(k) in November 2006 before resolving the FDA’s questions about our zirconia-toughened alumina product candidates. It is possible that the FDA could raise the same or similar questions about our current silicon nitride spinal product candidates, which could result in our having to perform additional studies. While we believe that our current product candidates incorporating silicon nitride ceramic materials differ significantly from our previous product candidates employing zirconia-toughened alumina, we cannot assure you that the FDA will not raise similar questions regarding our current spinal product candidates. If the FDA takes a similar position regarding our product candidates incorporating silicon nitride ceramic materials, our ability to bring our lead products to market could be delayed and we can give no assurance that we would ultimately receive marketing approval.
Even if the FDA permits us to use the 510(k) clearance process, we cannot assure you that the FDA will not require either supporting data from laboratory tests or studies that we have not conducted, or substantial supporting clinical data. If we are unable to use the 510(k) clearance process for any of our lead product candidates, are required to provide clinical data or laboratory data that we do not possess to support our 510(k) premarket notifications for any of these product candidates, or otherwise experience delays in obtaining or fail to obtain regulatory clearances, the commercialization of our most advanced product candidates will be delayed or prevented, which will adversely affect our ability to generate revenues. It also may result in the loss of potential competitive advantages that we might otherwise attain by bringing our products to market earlier than our competitors. Any of these contingencies could adversely affect our business.
Even if we succeed in obtaining FDA clearance or approvals for our lead product candidates and pipeline product candidates within the time frames we anticipate, our products may not be commercially successful.
Even if we receive regulatory clearances or approvals for our lead product candidates and pipeline product candidates, our product candidates may not gain market acceptance among orthopedic surgeons and the medical community. Orthopedic surgeons may elect not to use our products for a variety of reasons, including:
| • | lower than expected clinical benefits in comparison with other implant products; |
| • | surgeons’ perception that there are insufficient advantages of our implants relative to currently available implant products; |
| • | lack of coverage or adequate payment from managed care plans and other third-party payors for the procedures that use our products; |
| • | ineffective marketing and distribution support; |
| • | inadequate training of surgeons in the proper use of our products; |
| • | the development of alternative implant materials and products that render our products less competitive or obsolete; and |
| • | timing of the introduction of competitive products to market. |
If orthopedic surgeons do not perceive our implant products as attractive alternatives to existing products, we will not be able to generate significant revenues, if any.
9
The orthopedic implant market is highly competitive and we may not be able to compete effectively against the larger, well-established companies that dominate this market or emerging and small innovative companies that may seek to obtain or increase their share of the market.
The markets for spine, hip and knee implant products are intensely competitive, and many of our competitors are much larger and have substantially more financial and human resources than we do. Many have long histories and strong reputations within the industry, and a relatively small number of companies dominate these markets. For example, in 2006, Medtronic Spinal and Biologics, a subsidiary of Medtronic, Inc.; Synthes, Inc.; DePuy Spine, Inc., a subsidiary of Johnson & Johnson; Stryker Spine, a division of Stryker Corporation; Biomet Spine and Biomet Trauma, a subsidiary of Biomet, Inc.; and Zimmer Spine, a subsidiary of Zimmer Holdings, Inc., accounted for over 80% of spine implant sales worldwide. In the hip and knee implant market, Zimmer Holdings, Inc.; DePuy Orthopaedics, Inc., a subsidiary of Johnson & Johnson; Stryker Orthopaedics, a division of Stryker Corporation; Biomet, Inc.; and Smith & Nephew Orthopaedics, a subsidiary of Smith & Nephew plc, accounted for over 80% of sales worldwide.
These companies enjoy significant competitive advantages over us, including:
| • | broad implant product offerings, which address the needs of orthopedic surgeons and hospitals in a wide range of implant procedures; |
| • | greater experience in, and resources for, launching, marketing, distributing and selling products, including strong sales forces and established distribution networks; |
| • | existing relationships with spine and joint reconstruction surgeons; |
| • | more extensive intellectual property portfolios and resources for patent protection; |
| • | greater financial and other resources for product research and development; |
| • | greater experience in obtaining and maintaining FDA and other regulatory clearances or approvals for products and product enhancements; |
| • | established manufacturing operations and contract manufacturing relationships; |
| • | significantly greater name recognition and more recognizable trademarks; and |
| • | established relationships with healthcare providers and payors. |
Even if we successfully introduce implant products to market based on our ceramic materials, we may not succeed in overcoming the competitive advantages of these large and dominant orthopedic implant companies. In addition, emerging and small innovative companies may seek to increase their market share and they may later possess competitive advantages, which could also impact our business even if we successfully introduce implant products based on our ceramic materials. Moreover, many other companies are seeking to develop ceramic-based implant products, and these companies may introduce products which compete effectively against our products in terms of performance, price or both.
If we are unable to establish a sales and marketing infrastructure and enter into suitable arrangements with independent sales agencies, we will not be able to commercialize our product candidates.
Upon FDA clearance, we intend to market and sell our lead spinal products in the United States using a hybrid distribution network that includes a combination of experienced, independent sales agents with strong, existing surgeon relationships and a direct sales force in selected markets. A similar hybrid sales force will also be used to market our hip and knee reconstructive products. We have not yet established an internal sales organization, and we will need to recruit and train sales and marketing personnel in time for the launch of our most advanced product candidates, as well as expand our marketing capabilities as we grow our business. The establishment of our sales force will be expensive and time consuming, and we cannot assure you that we will be able to recruit and train a sufficient number of experienced and effective sales personnel on a timely basis.
10
In addition, we cannot assure you that we will succeed in entering into productive arrangements with an adequate number of sales agencies that are sufficiently dedicated to selling our products. The establishment of a network of sales agencies is expensive and time consuming. Furthermore, many potential sales agencies will market and sell the products of our competitors. Even if these sales agencies agree to market and sell our products, our competitors may be able, by offering higher commission payments or other incentives, to persuade these sales agencies to reduce or terminate their sales and marketing efforts of our products. Even if we enter into agreements with independent sales agencies, they may not generate revenue as quickly as we expect them to, commit the necessary resources to effectively market and sell our products, or ultimately succeed in selling our products. If we are not successful in building an effective external sales and marketing network to complement our internal sales force, we will have difficulty commercializing our product candidates, which would adversely affect our business and financial condition.
We are in the process of establishing our own certified manufacturing facility and validating of our manufacturing processes to produce our silicon nitride-based implant products, and we may not be successful in developing the necessary commercial-scale manufacturing processes, facilities and capabilities.
Prior to March 2006, we utilized an internal pilot manufacturing facility to produce prototypes of some of our ceramic product candidates, and we used third parties to produce components of some of our other ceramic product candidates, such as silicon nitride ball blanks for femoral heads. We are currently in the process of developing internal manufacturing operations, and we will need to continue our efforts to develop scaled-up processes, equip our facility and recruit and train manufacturing personnel before the commercial launch of our lead implant products. We anticipate our facility becoming fully operational for commercial-scale production by the end of 2007. Although we have received an International Standards Organization, or ISO, certification for our facility from the British Standards Institution, our facilities are yet to be inspected by the FDA. In addition to developing and working to scale-up a process for the manufacture of our silicon nitride ceramic products, we are also currently verifying a manufacturing process for our implant products that incorporate features of our CSC technology. We cannot assure you that we will be able to establish commercial-scale production of our products using cost-effective, reliable processes in facilities that meet applicable regulatory requirements. If we are unable to manufacture our products with consistent and satisfactory quality, at competitive costs, and sufficient quantities to meet demand, any of these circumstances may cause us to delay the introduction of our products or, once our products are introduced, may cause hospitals and surgeons to refrain from placing orders for them.
If we fail to comply with the FDA’s quality system regulation, the manufacture of our products could be delayed or interrupted and our products may be subject to product recalls.
We will be required to comply with the FDA’s quality system regulation, or QSR, which covers, among other things, the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. The FDA monitors compliance with the QSR through inspections of manufacturing facilities. If we are determined not to be in compliance or if any corrective action plan is not sufficient, we could be prevented or forced to delay the manufacture of our products, which could have a material adverse effect on our business, financial condition and results of operations. Moreover, after we have introduced products, any failure to maintain QSR compliance could force us to cease the manufacture of our products and subject us to other enforcement sanctions, including withdrawal of our products from the market, and delay or interrupt the manufacture of additional products.
We are in the process of developing a cost-effective process for the manufacture of our products based on our CSC technology, and if we are unable to implement such a process on a timely basis, we will experience delays in the introduction of our implant products that incorporate our CSC technology.
We are in the process of implementing an exclusively licensed process for the manufacture of our product candidates that will incorporate our CSC technology. We cannot assure you that we will succeed in our process implementation efforts for the manufacture of our product candidates that will incorporate our CSC technology. Delays in achieving a cost-effective and reliable process for commercial-scale production of implant products
11
that incorporate our CSC technology could impede the introduction of those product candidates and would adversely affect our business.
We depend on a limited number of third-party suppliers for key raw materials used in our manufacturing processes, and the loss of these third-party suppliers or their inability to supply us with adequate raw materials could harm our business.
We rely on a limited number of third-party suppliers for the raw materials required for the production of our implant products that will be made using silicon nitride, and we currently are developing arrangements with secondary sources for these raw materials. Our dependence on a limited number of third-party suppliers and the challenges we may face in obtaining adequate supplies of raw materials involve several risks, including limited control over pricing, availability, quality, and delivery schedules. We cannot be certain that our current suppliers will continue to provide us with the quantities of these raw materials that we require or satisfy our anticipated specifications and quality requirements. Any supply interruption in limited or sole sourced raw materials could materially harm our ability to manufacture our products until a new source of supply, if any, could be identified and qualified. Although we believe there are other suppliers of these raw materials, we may be unable to find a sufficient alternative supply channel in a reasonable time or on commercially reasonable terms. Any performance failure on the part of our suppliers could delay the development and commercialization of our implant products, including limiting supplies necessary for clinical trials and regulatory approvals, or interrupt production of then existing products that are already marketed, which would have a material adverse effect on our business.
If hospitals and other healthcare providers are unable to obtain coverage and adequate payments for procedures performed with our products, it is unlikely our products will be widely used.
Successful sales of our products will depend on the availability of coverage and adequate payments from third-party payors, including government programs such as Medicare and Medicaid, private insurance plans and managed care programs for procedures utilizing our future products. Hospitals and other healthcare providers that purchase orthopedic implant products for treatment of their patients generally rely on third-party payors to pay for all or part of the costs and fees associated with the procedures performed with or utilizing these devices. The existence of coverage and adequate payments for our products and the procedures performed with them by government and private insurance plans are central to acceptance of our lead and pipeline products. Many private payors currently base their reimbursement policies on the coverage decisions and payment amounts determined by the Centers for Medicare and Medicaid Services, or CMS, which administers the Medicare program. Others may adopt different coverage or payment policies for procedures performed with our products, while some governmental programs, such as Medicaid, have reimbursement policies that vary from state to state, some of which may not pay for the procedures performed with our products in an adequate amount, if at all. Our success may also be impacted by future action by CMS or other government agencies aimed at limiting payments to physicians, outpatient centers and hospitals. Additionally, as the portion of the U.S. population eligible for Medicare continues to grow, we will be more vulnerable to reimbursement limitations imposed by Medicare. For example, in 2006 CMS issued a national coverage decision denying Medicare coverage for DePuy’s CHARITE™ prosthetic intervertebral disc implant for patients over 60 years old. Also, the healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by paying service providers lower rates. Therefore, we cannot be certain that our products or the procedures performed with them will be covered or adequately reimbursed and thus we may be unable to sell our products profitably if third-party payors deny coverage or reduce their levels of payment below that which we project, or if our production costs increase at a greater rate than payment levels.
In addition, future reimbursement may be subject to international regulatory approval requirements and increased restrictions in international markets. Medical device regulatory requirements and healthcare payment systems vary significantly from country to country, and each country’s health care system may include both government sponsored healthcare and private insurance. Many countries have also instituted price ceilings on specific product lines. Any failure to receive regulatory and reimbursement approvals would negatively impact market acceptance of our products in any other international markets in which those approvals are sought.
12
We are dependent on our senior management team, engineering team, sales and marketing team and key surgeon advisors, and the loss of any of them could harm our business.
We have not entered into employment agreements with any of the members of our senior management team, and, therefore, there are no assurances that the services of any of these individuals will be available to us for any specified period of time. The loss of members of our senior management team, sales and marketing team, engineering team and key surgeon advisors, or our inability to attract or retain other qualified personnel or advisors could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Regulatory Approval of Our Products and Other Government Regulations
The safety of our products is not yet supported by any long-term clinical data, and they may prove to be less safe and effective than our laboratory data indicate.
We intend to seek clearance or approval for each of our lead and pipeline product candidates through the FDA’s 510(k) or PMA process depending on the product candidate. The 510(k) clearance process is based on the FDA’s agreement that a new product candidate is substantially equivalent to an already marketed product for which a PMA was not required and requires little or no additional supporting clinical data. Long-term clinical data or marketing experience obtained after clearance may indicate that our products cause unexpected complications or other unforeseen negative effects. If this happens, we could be subject to the withdrawal of our marketing clearance and other enforcement sanctions by the FDA, product recalls, significant legal liability, significant negative publicity, damage to our reputation and a dramatic reduction in our ability to sell our products, any one of which would have a material adverse effect on our business, financial condition and results of operations.
We expect to be required to conduct clinical trials for our pipeline product candidates. We have no experience conducting clinical trials, they may proceed more slowly than anticipated, and we cannot be certain that our products will be shown to be safe and effective for human use.
In order to commercialize our pipeline product candidates, we must submit a PMA for most of these product candidates, which will require us to conduct clinical trials. Even though we plan to seek FDA clearance of our pipeline Infinia Total Hip Implant product through the 501(k) process, the FDA has indicated that it expects us to conduct a clinical trial in support of our 510(k). We will receive approval from the FDA to commercialize pipeline products requiring a clinical trial only if we can demonstrate to the satisfaction of the FDA, in well-designed and properly conducted clinical trials, that our product candidates are safe and effective and otherwise meet the appropriate standards required for approval for specified indications. Clinical trials are complex, expensive, time consuming, uncertain and subject to substantial and unanticipated delays. Before we may begin clinical trials, we must submit and obtain approval for an investigational device exemption, or IDE, that describes, among other things, the manufacture of, and controls for, the device and a complete investigational plan. Clinical trials generally involve a substantial number of patients in a multi-year study. We may encounter problems with our clinical trials and any of those problems could cause us or the FDA to suspend those trials, or delay the analysis of the data derived from them.
A number of events or factors, including any of the following, could delay the completion of our clinical trials in the future and negatively impact our ability to obtain FDA approval for, and to introduce a particular pipeline product candidate:
| • | failure to obtain approval from the FDA or any foreign regulatory authority to commence an investigational study; |
| • | conditions imposed on us by the FDA or any foreign regulatory authority regarding the scope or design of our clinical trials; |
| • | delays in obtaining or in our maintaining required approvals from institutional review boards or other reviewing entities at clinical sites selected for participation in our clinical trials; |
13
| • | insufficient supply of our pipeline product candidates or other materials necessary to conduct our clinical trials; |
| • | difficulties in enrolling patients in our clinical trials; |
| • | negative or inconclusive results from clinical trials, or results that are inconsistent with earlier results, that necessitate additional clinical studies; |
| • | serious or unexpected side effects experienced by patients in whom our pipeline product candidates are implanted; or |
| • | failure by any of our third-party contractors or investigators to comply with regulatory requirements or meet other contractual obligations in a timely manner. |
Our clinical trials may not begin as planned, may need to be redesigned, and may not be completed on schedule, if at all. Delays in our clinical trials may result in increased development costs for our product candidates, which could cause our stock price to decline and limit our ability to obtain additional financing. In addition, if one or more of our clinical trials are delayed, competitors may be able to bring products to market before we do, and the commercial viability of our product candidates could be significantly reduced.
Once our products are commercialized, we and our independent sales agents must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause a material adverse effect on our business, financial condition and results of operations.
Once our products are commercialized, our relationships with surgeons, hospitals and the marketers of our products will become subject to scrutiny under various federal anti-kickback, self-referral, false claims and similar laws, often referred to collectively as healthcare fraud and abuse laws. Healthcare fraud and abuse laws are complex, and even minor, inadvertent violations can give rise to claims that the relevant law has been violated. Possible sanctions for violation of these fraud and abuse laws include monetary fines, civil and criminal penalties, exclusion from federal and state healthcare programs, including Medicare, Medicaid, Veterans Administration health programs, workers’ compensation programs and TRICARE (the healthcare system administered by or on behalf of the U.S. Department of Defense for uniformed services beneficiaries, including active duty and their dependents, retirees and their dependents), and forfeiture of amounts collected in violation of such prohibitions. Certain states in which we intend to market our products have similar fraud and abuse laws, imposing substantial penalties for violations. Any government investigation or a finding of a violation of these laws would likely result in a material adverse effect on the market price of our common stock, as well as our business, financial condition and results of operations.
Anti-kickback laws and regulations prohibit any knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for the referral of an individual or the ordering or recommending of the use of a product or service for which payment may be made by Medicare, Medicaid or other government-sponsored healthcare programs. We have entered into consulting agreements and product development agreements with surgeons, including some who may make referrals to us or order our products after our products are introduced to market. In addition, some of these surgeons own our stock, which they purchased in arms’ length transactions on terms identical to those offered to non-surgeons, or received stock options from us as consideration for consulting services performed by them. Other surgeons may be offered shares as part of this offering under our directed share program as described in the “Underwriters” section of this prospectus. While these transactions were structured with the intention of complying with all applicable laws, including the federal ban on physician self-referrals, commonly known as the “Stark Law,” state anti-referral laws and other applicable anti-kickback laws, it is possible that regulatory or enforcement agencies or courts may in the future view these transactions as prohibited arrangements that must be restructured or for which we would be subject to other significant civil or criminal penalties, or prohibit us from accepting referrals from these surgeons. Because our strategy relies on the involvement of surgeons who consult with us on the design of our product candidates, we could be materially impacted if regulatory or enforcement agencies or courts interpret our financial relationships with our surgeon
14
advisors who refer or order our products to be in violation of applicable laws and determine that we would be unable to achieve compliance with such applicable laws. This could harm our reputation and the reputations of our surgeon advisors. In addition, the cost of non-compliance with these laws could be substantial since we could be subject to monetary fines and civil or criminal penalties, and we could also be excluded from federally-funded healthcare programs, including Medicare and Medicaid, for non-compliance.
The scope and enforcement of all of these laws is uncertain and subject to rapid change, especially in light of the lack of applicable precedent and regulations. There can be no assurance that federal or state regulatory or enforcement authorities will not investigate or challenge our current or future activities under these laws. Any investigation or challenge could have a material adverse effect on our business, financial condition and results of operations. Any state or federal regulatory or enforcement review of us, regardless of the outcome, would be costly and time consuming. Additionally, we cannot predict the impact of any changes in these laws, whether these changes are retroactive or will have effect on a going-forward basis only.
We face significant uncertainty in the industry due to government healthcare reform.
Political, economic and regulatory influences are subjecting the healthcare industry to fundamental changes. Reforms under consideration in the United States include mandated basic healthcare benefits, controls on healthcare spending, increases in insurance premiums and increased out-of-pocket requirements for patients, the creation of large group purchasing organizations that aim to reduce the costs of products that their member hospitals consume, and significant modifications to the healthcare delivery system. We anticipate that the U.S. Congress and state legislatures will continue to review and assess alternative healthcare delivery systems and payment methods. Due to uncertainties regarding the ultimate features of reform initiatives and the timing of their enactment and implementation, we cannot predict which, if any, of such reform proposals will be adopted, when they may be adopted or what impact reform initiatives may have on us.
Risks Related to Our Intellectual Property and Litigation
If the combination of patents, trade secrets and contractual provisions that we rely on to protect our intellectual property is inadequate, our ability to commercialize our orthopedic implants successfully will be harmed, and we may not be able to operate our business profitably.
Our success depends significantly on our ability to protect our proprietary rights to the technologies incorporated in our products. We currently have four issued U.S. patents, twelve pending U.S. patent applications, and ten pending foreign patent applications. We rely on a combination of patent protection, trade secret laws and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these may not adequately protect our rights or permit us to gain or keep any competitive advantage.
The issuance of a patent is not conclusive as to its scope, validity or enforceability. The scope, validity or enforceability of our issued patents can be challenged in litigation or proceedings before the U.S. Patent and Trademark Office, or the USPTO. In addition, our pending patent applications include claims to numerous important aspects of our products under development that are not currently protected by any of our issued patents. We cannot assure you that any of our pending patent applications will result in the issuance of patents to us. The USPTO may deny or require significant narrowing of claims in our pending patent applications. Patents issued as a result of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is advantageous to us. Proceedings before the USPTO could result in adverse decisions as to the priority of our inventions and the narrowing or invalidation of claims in issued patents. The laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all.
15
Our competitors may successfully challenge and invalidate or render unenforceable our issued patents, including any patents that may issue in the future, which could prevent or limit our ability to market our products and could limit our ability to stop competitors from marketing products that are substantially equivalent to ours. In addition, competitors may be able to design around our patents or develop products that provide outcomes that are comparable to our products but that are not covered by our patents.
We also rely on an exclusive license from Dytech Corporation Ltd., or Dytech, for rights under three patents relating to a manufacturing process that can be used to implement our CSC technology. Our exclusive license from Dytech will be in effect for as long as we continue to have payment obligations to Dytech under the license, unless the license is earlier terminated on account of a continuing material violation of the license agreement. In the event of an early termination, we would not be able to rely on Dytech’s patents for the manufacturing process for the implementation of our CSC technology, and our ability to manufacture and commercialize our products incorporating this technology would be significantly impacted in an adverse manner.
Further, in the event that we are not able to commercialize a product or product candidate incorporating the licensed technology from Dytech within three years of the effective date of the agreement, or December 20, 2009 (or four years in the event clinical trials are required for FDA clearance, or December 20, 2010), Dytech will have the right, upon thirty days prior written notice to us, to convert the exclusive license into a non-exclusive license. In the event that our exclusive license is converted into a non-exclusive license, other competitors may be able to obtain licenses similar to ours that would substantially impair our ability to prevent competitors from commercializing products similar to ours.
We have also entered into confidentiality and assignment of intellectual property agreements with certain of our employees, consultants and advisors as one of the ways we seek to protect our intellectual property and other proprietary technology. However, these agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements.
In the event a competitor infringes upon one of our patents, our licensed patents or other intellectual property rights, enforcing our rights may be difficult, time consuming and expensive, and would divert management’s attention from managing our business. There can be no assurance that we will be successful on the merits in any enforcement effort. In addition, we may not have sufficient resources to litigate, enforce or defend our intellectual property rights.
We have no patent protection covering the composition of our formulation of silicon nitride or the process we use for manufacturing silicon nitride, and competitors may create doped-silicon nitride implant products substantially similar to ours, which could significantly diminish the effect of any competitive advantages that we might otherwise have had.
The composition of silicon nitride formulated with dopants such as yttira and alumina is generally known or is readily knowable, and we have no patent protection either for the composition of our formulation of silicon nitride, which we refer to as MC2, or for the process of manufacturing our MC2 silicon nitride and implant products made from that material. Moreover, we are aware of at least one ceramic manufacturer that already has the capability of manufacturing silicon nitride with strength, fracture resistance, and wear resistance characteristics similar to our MC2 silicon nitride. If other orthopedic companies decide to compete with us by manufacturing implants made from silicon nitride, or by marketing implants with silicon nitride components purchased from suppliers, we will have no ability to prevent them from doing so, except to the extent that specific implant embodiments are covered by our issued patents. To date, we have been issued one U.S. patent related to our MC2 technology, directed to the use of silicon nitride, with certain flexural strength and toughness characteristics, in the concave component of an articulating implant, where the convex component is made of a cobalt chromium metal alloy. Although we have submitted patent applications directed to other implant embodiments, such as an articulating implant where the concave component is made of a cobalt chromium metal alloy and the convex component is made of silicon nitride, or where both the concave and convex components are made of silicon
16
nitride, there is no assurance that such applications will issue as patents. If we fail to obtain patents with claims of a scope necessary to cover the various embodiments of orthopedic implants we intend to develop, our competitors will have the right to seek to develop and market substantially similar orthopedic implants made of silicon nitride. We are aware of one other company that appears to be developing at least one implant component made from silicon nitride, and we cannot assure you that our competitors will not seek to develop and market orthopedic implants made of silicon nitride in the future. The introduction by our competitors of orthopedic implants made from silicon nitride could negatively impact our ability to maintain a competitive advantage based on our MC2 technology, particularly if such competitive silicon nitride implants possessed strength, fracture resistance, wear resistance and imaging characteristics similar to our MC2 silicon nitride.
We continue to develop and refine our manufacturing processes to produce silicon nitride implants, and we believe we have already developed, and will continue to develop, significant know-how related to these processes. However, there is no assurance that we will be able to maintain this know-how as trade secrets, and competitors may develop or acquire equally valuable or more valuable know-how related to the manufacture of silicon nitride implants. Further, if any of our competitors is able to obtain patent protection for its composition of silicon nitride or for its process for manufacturing silicon nitride, we could become subject to patent infringement claims and further be prevented from continuing the manufacture of our silicon nitride based implant products.
We could become subject to intellectual property litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages, prevent us from marketing our product candidates under development, and/or reduce the margins we may realize from our products that we may commercialize.
The medical devices industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, and the determination is often uncertain. There may be existing patents of which we are unaware that our products under development may inadvertently infringe. The likelihood that patent infringement claims may be brought against us increases as the number of participants in the market for spine, hip and knee implants increases and as we achieve more visibility in the market place and introduce products to market.
Any infringement claim against us, even if without merit, may cause us to incur substantial costs, and would place a significant strain on our financial resources, divert the attention of management from our core business, and harm our reputation. In some cases, litigation may be threatened or brought by a patent holding company or other adverse patent owner who has no relevant product revenues and against whom our patents may provide little or no deterrence. If we were found to infringe any patents, we could be required to pay substantial damages, including triple damages if an infringement is found to be willful, and royalties and could be prevented from selling our products unless we obtain a license or are able to redesign our products to avoid infringement. We may not be able to obtain a license enabling us to sell our products on reasonable terms, or at all, and there can be no assurance that we would be able to redesign our products in a way that would not infringe those patents. If we fail to obtain any required licenses or make any necessary changes to our technologies or the products that incorporate them, we may be unable to commercialize one or more of our products or may have to withdraw products from the market, all of which would have a material adverse effect on our business, financial condition and results of operations.
In addition, in order to further our product development efforts, we have entered into agreements with orthopedic surgeons to help us design and develop new products, and we expect to enter into similar agreements in the future. In certain instances, we have agreed to pay such surgeons royalties on sales of products which incorporate their product development contributions. There can be no assurance that surgeons with whom we have entered into such arrangements will not claim to be entitled to a royalty even if we do not believe that such products were developed by cooperative involvement between us and such surgeons. In addition, some of our surgeon advisors have agreements with other orthopedic companies pursuant to which they have agreed to assign to those other companies their rights in inventions which they conceive or develop, or help conceive or develop.
17
There can be no assurance that one or more of these orthopedic companies will not claim ownership rights to an invention we develop in collaboration with our surgeon advisors or consultants on the basis that an agreement with such orthopedic company gives it ownership rights in the invention. Any such claim against us, even without merit, may cause us to incur substantial costs, and would place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation.
If our ceramic technologies or our product candidates conflict with the rights of others, we may not be able to manufacture or market our product candidates, which could have a material and adverse effect on us.
Our commercial success will depend in part on not infringing the patents or violating the other proprietary rights of third parties. We are aware of an issued patent that was recently granted to DePuy by the European Patent Office (EP 1212013) based on international patent application no. WO 01/17464, entitled “Combination of Material for Joint Prosthesis” (the “EP ’013 patent”). The EP ’013 patent was granted for nineteen different designated European states and claims an orthopedic joint prosthesis having metal on ceramic articulating surfaces, wherein the hardness of the metallic material is at least about 2500 MPa, and the hardness of the ceramic material is greater than that of the metallic material by at least about 4000 MPa, where the articulating surfaces have sphericity not more than 0.01 microns and surface roughness more than 0.05 microns.
We are aware that several third parties, including Biomet UK Ltd, filed post-grant oppositions to the EP ’013 patent raising objections as to its scope and validity. An opposition is a proceeding that allows third parties to challenge the validity of a European patent granted by the European Patent Office. The European Patent Office recently dispatched a communication indicating that DePuy had informed the European Patent Office that it no longer approved the text in the form that EP ’013 was granted and would not approve an alternative text, requesting on this basis that the patent should be revoked. As a result, the Opposition Division of the European Patent Office has now revoked the patent. However, we are aware that a divisional application, based on the EP ’013, is still pending before the European Patent Office, and accordingly, there can be no assurances that DePuy may not have filed, or will not in the future file another divisional patent application with a similar scope to the EP ’013 patent. We are monitoring the situation closely. If another pending or a new divisional patent application results in the issuance by the European Patent Office of a patent similar in scope, and if our activities are determined to be covered by such a patent, we cannot provide any assurance that DePuy would be willing to grant us a license on terms we or they would consider commercially reasonable, if at all. As a consequence, we could be prevented from manufacturing and marketing high-strength ceramic-on-metal articulating implants in the Europe, which would have a material adverse effect on our business, financial condition and results of operations.
The EP ’013 patent had a corresponding U.S. counterpart patent application pending in the United States involving similar claims (U.S. Pub. 2005/0033442). The U.S. patent office recently rejected all of these claims, and then declared the application to be abandoned because DePuy did not respond to the rejection. There can be no assurances that DePuy has not filed, or will not file in the future, a petition to revive the abandoned U.S. application. Such a petition would only be granted by the patent office if DePuy’s abandonment of the U.S. application was unintentional or unavoidable. DePuy may also have other pending U.S. patent applications of which we are unaware directed to the same or similar subject matter as the abandoned application. We are monitoring this situation in the United States closely.
Issued patents held by others may limit our ability to develop commercial products. All issued patents are entitled to a presumption of validity under the laws of the United States. If we need suitable licenses to such patents to permit us to develop or market our product candidates, we may be required to pay significant fees or royalties and we cannot be certain that we would even be able to obtain such licenses. Competitors or third parties may obtain patents that may cover subject matter we use in developing the technology required to bring our products to market, that we use in producing our products, or that we use in treating patients with our products. We know that others have filed patent applications in various jurisdictions that relate to several areas in which we are developing products. Some of these patent applications have already resulted in patents and some
18
are still pending. If we were found to infringe any of these issued patents or any of the pending patent applications, when and if issued, we may be required to alter our processes or product candidates, pay licensing fees or cease activities. If use of technology incorporated into or used to produce our product candidates is challenged, or if our processes or product candidates conflict with patent rights of others, third parties could bring legal actions against us, in Europe, the United States and elsewhere, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. Additionally, it is not possible to predict with certainty what patent claims may issue from pending applications. In the United States, for example, patent prosecution can proceed in secret prior to issuance of a patent, provided such application is not filed in foreign jurisdiction. For U.S. patent applications that are also filed in foreign jurisdictions, such patent applications will not publish until 18 months from the filing date of the application. As a result, third parties may be able to obtain patents with claims relating to our product candidates which they could attempt to assert against us. Further, as we develop our products, third parties may assert that we infringe the patents currently held or licensed by them and we cannot predict the outcome of any such action.
There has been extensive litigation in the medical devices industry over patents and other proprietary rights. If we become involved in any litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license, grant cross-licenses and pay substantial royalties in order to continue to manufacture or market the affected products.
We cannot assure you that we would prevail in any legal action or that any license required under a third party patent would be made available on acceptable terms or at all. Ultimately, we could be prevented from commercializing a product, or forced to cease some aspect of our business operations, as a result of claims of patent infringement or violation of other intellectual property rights, which could have a material and adverse effect on our business, financial condition and results of operations.
We could be prevented from using the trademark “Amedica” as our name and could be prevented from using “Amedica” in conjunction with our products, which could eliminate any goodwill associated with “Amedica” and negatively impact the marketing of our products.
We applied for but have not yet received a registration in the United States for the trademark “Amedica”. Another company, Amedica Biotech, has an earlier filed, trademark registration for the same mark. Amedica Biotech registered its mark for use in commerce with certain types of pharmaceutical products. Though we are seeking registration for use in connection with medical apparatuses, if the U.S. Patent and Trademark Office finds that we were not the first to use the mark in commerce and that a likelihood of confusion exists, we may not be able to register our mark. Further, Amedica Biotech may seek to enjoin our use of the mark. If Amedica Biotech is successful, we would have to change our name, which could be an expensive and time-consuming process, and we would lose any goodwill we have thus far created in the mark.
We also filed an application on August 31, 2006 to register “Amedica” with the Office for Harmonization of the Internal Market, which is the trademark and industrial design registry of the European Union. We have learned that an opposition to this application was filed on February 26, 2007, by Addmedica (S.A.S.), or Addmedica, a French company, claiming a likelihood of confusion. The opposition was deemed admissible on March 9, 2007. Through July 11, 2007, we were in a “cooling off period” during which we and Addmedica had the opportunity to reach a negotiated settlement. The cooling-off period was not extended and either party may proceed with an adversarial opposition. We can negotiate a settlement with Addmedica at any time until the Office for Harmonization of the Internal Market reaches a final decision, which will be after November 11, 2007. In order to reach a settlement with Addmedica, we may be forced to make concessions such as altering the European labeling of our products, restricting the European channels of distribution we plan to use to market our products, narrowing the description of goods and services in our pending application, or limiting the geographic markets we seek to operate in within Europe, any of which could have an adverse effect on our operations. If we are unable to reach a negotiated settlement and the opposition is successful, we could be prevented from using “Amedica” in commerce in the European Union. This could require us to market our products under different
19
names in the European Union and the United States. This could adversely impact our efforts to achieve cross- border name recognition for our products, lead us to select a different trademark for the marketing of our products worldwide or to change our company name.
We may be subject to damages resulting from claims that we, our employees, or our independent sales agencies have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition agreements with our competitors or non-solicitation agreements.
Many of our employees were previously employed at other orthopedic implant companies, including our competitors and potential competitors. Many of our potential distributors sell, or in the past have sold, products of our competitors. We may be subject to claims that either we, or these employees or distributors, have inadvertently or otherwise used or disclosed the trade secrets or other proprietary information of our competitors. In addition, we have been and may in the future be subject to claims that we caused an employee or sales agent to break the terms of his or her non-competition agreement or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money damages, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to commercialize products, which could have an adverse effect on our business, financial condition and results of operations.
Risks Related to Potential Litigation from Operating Our Business
If we successfully commercialize our products under development, we will become subject to potential product liability claims, and we may be required to pay damages that exceed our insurance coverage.
We expect that our business will expose us to potential product liability claims that are inherent in the design, testing, manufacture, sale and distribution of orthopedic implants. Spine, hip and knee implants involve significant risks of serious complications, including bleeding, nerve injury, paralysis, infection, and even death. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or in our inability to secure coverage in the future on commercially reasonable terms, if at all. In addition, if our product liability insurance proves to be inadequate to pay a damage award, we may have to pay the excess of this award out of our cash reserves, which could significantly harm our financial condition. If longer-term patient results and experience indicate that our products or any component of a product causes tissue damage, motor impairment or other adverse effects, we could be subject to significant liability. A product liability claim, even one without merit, could harm our reputation in the industry, lead to significant legal fees, and result in the diversion of management’s attention from managing our business.
Any claims relating to our improper handling, storage or disposal of biological or hazardous materials could be time consuming and costly.
Although we do not believe that the manufacture of our ceramic implant products will involve the use of hazardous materials, it is possible that regulatory authorities may disagree or that changes to our manufacturing processes may result in such use. Our business and facilities and those of our suppliers and future suppliers may therefore be subject to foreign, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We may incur significant expenses in the future relating to any failure to comply with environmental laws. Any such future expenses or liability could have a significant negative impact on our business, financial condition and results of operations.
20
Risks Related to Our Need for Financing
We will require substantial additional financing and our failure to obtain additional funding when needed could force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We will require substantial future capital in order to continue to conduct the research and development and regulatory clearance and approval activities necessary to bring our products to market and to establish effective marketing and sales capabilities. We do not expect our existing capital resources and the net proceeds from this offering to be sufficient to enable us to fund the completion of the development and commercialization of all of our product candidates. We expect that our existing capital resources and the net proceeds from this offering will enable us to maintain currently planned operations through the end of 2009. However, our operating plan may change, and we may need additional funds sooner than anticipated to meet our operational needs and capital requirements for product development, clinical trials and commercialization.
We currently have no committed sources of capital. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more of our product candidates, or delay establishment of sales and marketing capabilities or other activities necessary to commercialize our product candidates.
Our future capital requirements will depend on many factors, including:
| • | the scope, progress, results and cost of our product development efforts; |
| • | the costs, timing and outcomes of regulatory reviews of our implant products; |
| • | the number and types of implant products we develop and commercialize; |
| • | the costs of establishing sales and marketing infrastructure and of establishing commercial-scale manufacturing operations; |
| • | the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims; |
| • | our ability to establish strategic collaborations or other arrangements on terms acceptable to us, the amount and timing of our expenditures, and our collaborators’ contributions under such arrangements; and |
| • | the extent and scope of our general and administrative expenses. |
Raising additional capital by issuing securities or through licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or products or grant licenses on terms that are not favorable to us. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and have a material adverse effect on our business, financial condition and results of operations.
We have incurred losses since our inception and anticipate that we will continue to incur substantial losses for the foreseeable future. We may never achieve or sustain profitability.
We have a limited operating history, have never generated any revenue from product sales and have incurred substantial net losses since our inception in 1996. As of March 31, 2007, we had an accumulated deficit of $18.2
21
million. Our net loss was $6.2 million for the year ended December 31, 2006, and $5.6 million for the three months ended March 31, 2007. Our losses have resulted principally from costs incurred in connection with our research and development activities and from general and administrative expenses associated with our operations. We have not yet commercialized any products, we do not expect to introduce any of our lead product candidates until 2008 and we do not anticipate introducing one of our lead spinal spacer product candidates until the second half of 2008. We do not expect to start clinical trials of the earliest of our pipeline products before the first half of 2009. Until we receive FDA clearances for our lead implant products and successfully launch those products, we expect to continue to incur substantial losses for the foreseeable future. We also expect our research and development expenses and general and administrative expenses to increase substantially following the completion of this offering as our products advance through the development cycle and we expand our infrastructure. As a result, we will need to generate significant revenues to pay these expenses and achieve profitability.
If we are unable to develop and commercialize any of our product candidates, if development is delayed, or if sales revenue from any product that receives marketing approval is insufficient, we may never become profitable. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis.
Risks Related to Our Common Stock and this Offering
There has been no prior public market for our common stock and an active trading market may not develop.
Prior to this offering, there has been no public market for our common stock. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market on The NASDAQ Global Market or otherwise or how liquid that market might become. The lack of an active market may impair the value of your shares and your ability to sell your shares at the time you wish to sell them. An inactive market may also impair our ability to raise capital by selling our common stock and may impair our ability to acquire other companies, products or technologies by using our common stock as consideration.
We expect that the price of our common stock will fluctuate substantially and you may not be able to sell your shares at or above the offering price.
You should consider an investment in our common stock risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The initial public offering price for the shares of our common stock sold in this offering will be determined by negotiation between the underwriters and us based on several factors. This price may not reflect the market price of our common stock following this offering. You may be unable to sell your shares of common stock at or above the initial public offering price due to fluctuations in the market price of our common stock arising from changes in our operating performance or prospects. In addition, the volatility of orthopedic implant company stocks often does not correlate to the operating performance of the companies represented by such stocks. Some of the factors that may cause the market price of our common stock to fluctuate include:
| • | our ability to develop, obtain regulatory clearances or approvals for, and market new and enhanced implant product candidates on a timely basis; |
| • | changes in governmental regulations or in the status of our regulatory approvals, clearances or future applications; |
| • | our announcements or our competitors’ announcements regarding new products, product enhancements, significant contracts, number and productivity of distributors, number of hospitals and surgeons using products, acquisitions or strategic investments; |
| • | announcements of technological or medical innovations for the treatment of orthopedic pathology; |
| • | delays or other problems with the manufacturing of our implant product candidates and related instrumentation; |
22
| • | volume and timing of orders for our product candidates, if and when commercialized; |
| • | changes in the availability of third-party reimbursement in the United States and other countries; |
| • | quarterly variations in our or our competitors’ results of operations; |
| • | changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock; |
| • | failure to meet estimates or recommendations by securities analysts, if any, who cover our stock; |
| • | changes in healthcare policy in the United States and internationally; |
| • | product liability claims or other litigation involving us; |
| • | sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; |
| • | disputes or other developments with respect to intellectual property rights; |
| • | changes in accounting principles; and |
| • | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit regardless of the outcome. Such a lawsuit also would divert the time and attention of our management.
Securities analysts may not initiate coverage of our common stock or may issue negative reports, which may have a negative impact on the market price of our common stock.
Securities analysts may elect not to provide research coverage of our common stock after the completion of this offering. If securities analysts do not cover our common stock after the completion of this offering, the lack of research coverage may cause the market price of our common stock to decline. The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about our business. If one or more of the analysts who elect to cover us downgrade our stock, our stock price would likely decline rapidly. If one or more of these analysts cease coverage of us, we could lose visibility in the market, which in turn could cause our stock price to decline. In addition, recently adopted rules mandated by the Sarbanes-Oxley Act and a global settlement reached in 2003 among the Securities and Exchange Commission, or the SEC, other regulatory agencies and a number of investment banks have led to a number of fundamental changes in how analysts are reviewed and compensated. In particular, many investment banking firms are required to contract with independent financial analysts for their stock research. It may be difficult for a company such as ours, with a smaller market capitalization, to attract independent financial analysts that will cover our common stock. This could have a negative effect on the market price of our stock.
If our executive officers, directors and principal stockholders choose to act together, they will be able to exert significant influence over us and our significant corporate decisions and may act in a manner that advances their best interests and not necessarily those of other stockholders.
Upon completion of this offering, our executive officers, directors, and beneficial owners of 5% or more of our outstanding common stock and their affiliates will beneficially own approximately 27.0% of our outstanding common stock, or approximately 25.8% if the underwriters’ over-allotment option is exercised in full. As a result, these persons, acting together, will have the ability to influence significantly the outcome of all matters
23
requiring stockholder approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including investors in this offering, by, among other things:
| • | delaying, deferring or preventing a change in control of us; |
| • | entrenching our management and/or our board of directors; |
| • | impeding a merger, consolidation, takeover or other business combination involving us; |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us; or |
| • | causing us to enter into transactions or agreements that are not in the best interests of all stockholders. |
We also plan to reserve up to 5.0% of the shares offered in this offering under a directed share program in which our executive officers and directors, principal stockholders, employees, business associates and related persons may be able to purchase shares in this offering at the initial public offering price. This program may further increase the percentage of stock held by persons whose interests are aligned with the interests of our executive officers, directors and principal stockholders.
Our management team may allocate the proceeds of this offering in ways in which you may not agree.
We intend to use the net proceeds from this offering to fund our product development efforts and the clearance or approval and subsequent commercialization of our product candidates; to establish our hybrid sales and marketing organization; to scale-up our manufacturing operations, continue to build out and equip our manufacturing facilities and to recruit and train manufacturing personnel; to support our research and development efforts; and for general corporate purposes. For a further description of our intended use of the net proceeds of this offering, see the “Use of Proceeds” section of this prospectus.
Because of the number and variability of factors that will determine our use of the net proceeds from this offering, our ultimate use of these proceeds may vary substantially from their currently intended use. Our management will have considerable discretion over the use of the net proceeds of this offering. Stockholders may not agree with such uses, and the net proceeds may be used in a manner that does not increase our operating results or market value.
Future sales of our common stock in the public market after this offering may cause our stock price to decline and impair our ability to raise future capital through the sale of our equity securities.
Upon completion of this offering, our current stockholders will hold a substantial number of shares of our common stock that they will be able to sell in the public market in the near future. Sales by our current stockholders of a substantial number of shares after this offering could significantly reduce the market price of our common stock. Moreover, following the completion of this offering, the holders of 1,457,830 shares of common stock, assuming the conversion of our convertible preferred stock, and holders of warrants to purchase 1,062,067 shares of common stock, assuming the conversion of preferred stock warrants into common stock warrants, will have rights, subject to some conditions, to require us to include their shares in registration statements that we may file for ourselves or other stockholders. These shares of common stock, totaling 2,519,897 shares, assuming the exercise of the common stock warrants, represent approximately 16.5% of the total number of shares of our common stock to be outstanding immediately after this offering, assuming conversion of the preferred stock warrants but no exercise of the underwriters’ over-allotment option. Please see the “Description of Capital Stock—Registration Rights” section of this prospectus for a description of the registration rights of these stockholders. In addition, immediately upon completion of this offering, approximately 4,853,796 and 4,433,768 shares of our outstanding common stock then held by existing stockholders which are deemed to be “restricted securities” pursuant to Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, will be eligible for sale in reliance on Rule 144(k) and Rule 144,
24
respectively, subject to the lock-up agreements described in the “Underwriters” section of this prospectus. Upon completion of this offering, a holder of warrants to acquire shares of our common stock will be able to net exercise such warrants by surrendering a portion of that holder’s warrants as payment of the exercise price rather than paying the exercise price in cash. As of June 30, 2007, warrants to acquire approximately 449,579 and 546,137 shares of our common stock would be eligible to rely upon on Rule 144(k) and Rule 144, respectively, if they are net exercised, all of which are subject to the lock-up agreements.
We also intend to register all shares of our common stock that we may issue pursuant to our 2003 Stock Option Plan and our 2007 Stock Plan. Shares issued by us upon exercise of options granted under our stock plans would be eligible for sale in the public market upon the effective date of the registration statement for those shares, subject to the lock-up agreements described in the “Underwriters” section of this prospectus. If any of these holders cause a large number of securities to be sold in the public market, the sales could reduce the trading price of our common stock. These sales also could impede our ability to raise future capital. Please see the “Shares Eligible for Future Sale” section of this prospectus for a description of sales that may occur in the future.
We will incur increased costs as a result of changes in laws and regulations relating to corporate governance matters.
As a public reporting company, we will need to comply with the Sarbanes-Oxley Act of 2002 and the related rules and regulations adopted by the SEC and by The NASDAQ Global Market, including expanded disclosures, accelerated reporting requirements and more complex accounting rules. Compliance with Section 404 of the Sarbanes-Oxley Act of 2002 and other requirements will increase our costs and require additional management resources. Additionally, these laws and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We are presently evaluating and monitoring developments with respect to these laws and regulations and cannot predict or estimate the amount or timing of additional costs we may incur to respond to their requirements.
Anti-takeover provisions in our organizational documents and Delaware law may discourage or prevent a change of control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon the completion of this offering contain provisions that could discourage, delay or prevent a merger, acquisition or other change of control of our company or changes in our board of directors that our stockholders might consider favorable, including transactions in which you might receive a premium for your shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove management. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| • | authorize our board of directors to create and issue, without prior stockholder approval, preferred stock that may have rights senior to those of our common stock and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors; |
| • | provide for a classified board of directors, such that not all members of our board will be elected at one time; |
25
| • | prohibit our stockholders from filling board vacancies, limit who may call stockholder meetings, and prohibit the taking of stockholder action by written consent; |
| • | prohibiting our stockholders from making certain changes to our amended and restated certificate of incorporation or amended and restated bylaws except with the approval of holders of 75% of the outstanding shares of our capital stock entitled to vote; and |
| • | require advance written notice of stockholder proposals that can be acted upon at stockholders meetings and director nominations to our board of directors. |
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
Investors in this offering will pay a much higher price than the book value of our common stock and, therefore, you will incur immediate and substantial dilution of your investment.
If you purchase common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. You will incur immediate and substantial dilution of $8.41 per share, representing the difference between the initial public offering price per share of our common stock and our pro forma net tangible book value per share after giving effect to this offering at the assumed initial public offering price of $14.00 per share of our common stock. In the past, we also issued options and warrants to acquire common stock at prices significantly below the assumed initial public offering price. To the extent these outstanding options are ultimately exercised, you will sustain further dilution. For a further description of the dilution you will incur in this offering, see the “Dilution” section of this prospectus.
We do not intend to pay cash dividends.
We have never declared or paid cash dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain all available funds and any future earnings for use in the operation and expansion of our business. In addition, the terms of any future debt or credit facility may preclude us from paying any dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of potential gain in your investment for the foreseeable future.
26
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to us. The forward-looking statements are contained principally in, but not limited to, the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | our ability to market, commercialize and achieve market acceptance of any of our product candidates that we are developing or may develop in the future; |
| • | our ability to become a profitable orthopedics implant company; |
| • | our ability to succeed in obtaining FDA clearance for our lead product candidates or FDA approvals for our pipeline product candidates; |
| • | the timing, costs and other limitations involved in obtaining regulatory clearance or approval for any of our product candidates and, thereafter, continued compliance with governmental regulation of our then existing products and activities; |
| • | our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; |
| • | our ability to obtain sufficient quantities and satisfactory quality of raw materials to meet our manufacturing needs; |
| • | the availability of adequate coverage reimbursement from third-party payors in the United States; |
| • | our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements, liquidity and our needs for additional financing; |
| • | our ability to establish a sales and marketing infrastructure and enter into suitable arrangements with independent sales agencies; |
| • | our ability to scale-up our manufacturing capabilities and facilities and become fully operational for commercial-scale production; |
| • | our ability to develop effective and cost efficient manufacturing processes for our products; |
| • | the safety and efficacy of our product candidates; |
| • | the timing of and our ability to conduct clinical trials; |
| • | our use of the proceeds of this offering; |
| • | potential changes to the healthcare delivery systems and payment methods by Congress and certain state legislatures; |
| • | any potential requirement by regulatory agencies that we restructure our relationships with referring surgeons; |
| • | our ability to develop and maintain relationships with surgeons, hospitals and marketers of our implants; and |
| • | our ability to attract and retain a qualified management team, engineering team, sales and marketing team, key surgeon advisors and other qualified personnel and advisors. |
27
In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” and elsewhere in this prospectus. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements.
Any forward-looking statement in this prospectus reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, industry and future growth. Except as required by law, we assume no obligation to update or revise any forward-looking statements contained in this prospectus, whether as a result of new information, future events or otherwise. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act do not protect any forward-looking statements that we make in connection with this offering.
28
We estimate that we will receive approximately $57.8 million in net proceeds from the sale of 4,650,000 shares of common stock that we are offering, or approximately $66.9 million if the underwriters exercise their over-allotment option in full, based upon the assumed initial public offering price of $14.00 per share, the midpoint of the range on the front cover of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 per share increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) the net proceeds to us from this offering by $4.3 million, or approximately $5.0 million if the underwriters exercise their over-allotment option in full, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to fund the development and commercialization of our lead products, to build our sales, marketing and distribution capabilities, to establish commercial-scale manufacturing operations, to fund research and development activities for our pipeline product candidates, to increase our working capital, to create a public market for our common stock, to increase our ability to access the capital markets in the future, to provide liquidity for our existing stockholders, and for general corporate purposes.
We currently expect to use the net proceeds from this offering in the following manner:
| • | approximately $3.0 million to fund the development and commercialization of our lead product candidates; |
| • | approximately $22.0 million to fund research and development and commercialization activities of our pipeline product candidates; |
| • | approximately $10.0 million to build sales, marketing and distribution capabilities for spine and reconstructive implants including the costs of instrumentation sets; and |
| • | the remainder for working capital and other general corporate purposes. |
In addition, we may use a portion of our net proceeds to introduce our spine, hip and knee implant products into selected international markets. Also, while we have no present understandings, commitments, or agreements to enter into any potential acquisitions, a portion of the net proceeds may also be used to acquire or invest in complementary businesses, technologies, services or products.
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. The amount and timing of our actual expenditures may vary significantly depending upon numerous factors, including the ultimate resolution of our FDA submissions for clearances or approvals of our product candidates, the specific clinical trial requirements imposed for market approval of our pipeline product candidates, our revenues, operating costs and capital expenditures, and other factors described under “Risk Factors.” We may find it necessary or advisable to use the net proceeds for other purposes, and our management will retain broad discretion in the allocation of our net proceeds from this offering.
Pending use of our net proceeds from this offering, we plan to invest the proceeds in a variety of capital preservation investments, including investment-grade, interest-bearing instruments. We cannot predict whether the net proceeds will yield a favorable return.
As of March 31, 2007, we had cash, cash equivalents and marketable securities of approximately $10.3 million. We believe that the net proceeds we raised in our Series D convertible preferred stock offering, the net proceeds from this offering, together with our cash and cash equivalent balances and interest we earn on these balances, will be sufficient to meet our anticipated cash requirements through the end of 2009. We will need to raise substantial additional funds before we can expect to commercialize all of our product candidates. We may satisfy our future cash needs through the sale of equity securities, debt financings, working capital lines of credit, corporate collaborations or license agreements, grant funding, or through interest income earned on cash balances.
29
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business.
30
The table below reflects our unaudited capitalization as of March 31, 2007:
| • | on an actual basis; |
| • | on a pro forma basis giving effect to (i) the issuance of 4,456,500 shares of our Series D convertible preferred stock and warrants exercisable for 253,290 shares of our Series D convertible preferred stock in April 2007, for aggregate net proceeds of approximately $12.4 million, (ii) the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 8,339,296 shares of our common stock upon the completion of this offering, and (iii) the conversion of all outstanding warrants exercisable for shares of our convertible preferred stock into warrants exercisable for a total of 1,062,067 shares of common stock (but not assuming the exercise of these common stock warrants), upon completion of this offering and the related reclassification of the preferred stock warrant liability to additional paid in capital; and |
| • | on a pro forma basis, as adjusted to give effect to the sale of 4,650,000 shares of common stock in this offering at an assumed initial public offering price of $14.00 per share (the midpoint of the range on the front cover of this prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses. |
You should read this table together with “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
| As of March 31, 2007 | ||||||||||||
| (unaudited) | ||||||||||||
| Actual | Pro Forma | Pro Forma as Adjusted |
||||||||||
| Long-term debt (net of current) |
$ | 1,137,625 | $ | 1,137,625 | $ | 1,137,625 | ||||||
| Preferred stock warrant liability |
6,304,963 | — |
|
— |
| |||||||
| Convertible preferred stock (consisting of Series A, Series B, Series C convertible preferred stock on an aggregated basis), $0.01 par value; 40,000,000 shares authorized, 27,400,058 shares issued and outstanding actual, and no shares issued and outstanding, pro forma and pro forma as adjusted |
|
26,389,982 |
|
— |
|
— |
| |||||
| Stockholders’ equity (deficit): |
||||||||||||
| Common stock, $0.01 par value; 60,000,000 shares authorized, 2,271,986 shares issued and outstanding actual, 10,611,282 shares issued and outstanding pro forma; 15,261,282 shares issued and outstanding pro forma as adjusted |
|
22,719 |
|
|
106,113 |
|
|
152,613 |
| |||
| Additional paid-in-capital |
|
591,632 |
|
|
45,603,183 |
|
|
103,349,683 |
| |||
| Deficit accumulated during the development stage |
(18,174,317 | ) | (18,174,317 | ) |
|
(18,174,317 |
) | |||||
| Total stockholders’ equity (deficit) |
(17,559,966 | ) |
|
27,534,979 |
|
|
85,327,979 |
| ||||
| Total capitalization(1) |
$ |
16,272,604 |
|
$ |
28,672,604 |
|
$ |
86,465,604 |
| |||
| (1) | As of March 31, 2007, our cash, cash equivalents and marketable securities on an actual basis, pro forma basis and pro forma as adjusted basis were $10.3 million, $22.7 million and $80.5 million, respectively. Cash, cash equivalents and marketable securities are indications of liquidity and do not constitute capitalization. |
A $1.00 per share increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) each of additional paid-in-capital, total stockholders’ equity (deficit) and total capitalization by approximately $4.3 million, assuming that the number of shares offered by us, as set forth on the cover page
31
of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma information discussed above is illustrative only and following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
The outstanding share information set forth above is as of March 31, 2007, and excludes:
| • | 905,198 shares of common stock issuable upon the exercise of outstanding options to purchase common stock, at a weighted average exercise price of $1.52 per share; |
| • | 1,062,067 shares of common stock issuable upon the exercise of warrants for shares of Series A, Series B, Series C and Series D convertible preferred stock, on an as-converted basis, at a weighted average exercise price of $5.20 per share; |
| • | 224,327 additional shares of common stock reserved for issuance under our 2003 Stock Option Plan; and |
| • | 2,000,000 additional shares of common stock for issuance under our 2007 Stock Plan, which becomes effective upon completion of this offering. |
32
If you invest in our common stock, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma net tangible book value per share of our common stock after this offering. We calculate net tangible book value per share by dividing the net tangible book value, or tangible assets less total liabilities, by the number of outstanding shares of common stock.
Our historical net tangible book value as of March 31, 2007 was a deficit of $17,559,966 or $7.73 per share of common stock. Our pro forma net tangible book value at March 31, 2007 was $27,534,979, or $2.59 per share, based on 10,611,282 shares of our common stock outstanding after giving effect to the conversion of all outstanding shares of our preferred stock, including 4,456,500 shares of our Series D convertible preferred stock issued in April 2007, into 8,339,296 shares of common stock and the conversion of 4,057,040 preferred stock warrants into 1,062,067 common stock warrants, including warrants to purchase a total of 253,290 shares of Series D convertible preferred stock issued in April 2007, and the related reclassification of the preferred stock warrant liability to additional paid in capital, upon the closing of this offering. After giving effect to the sale of 4,650,000 shares of common stock by us at an assumed initial public offering price of $14.00 per share, less the estimated underwriting discounts and commissions and our estimated offering expenses, our pro forma net tangible book value at March 31, 2007 would be $85.3 million, or $5.59 per share. This represents an immediate increase in the pro forma net tangible book value of $3.00 per share to existing stockholders and an immediate dilution of $8.41 per share to new investors purchasing shares at an assumed initial public offering price of $14.00 per share. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | 14.00 | |||||
| Actual net tangible deficit per share as of March 31, 2007 |
$ | (7.73 | ) | ||||
| Pro forma increase per share attributable to conversion of preferred stock and preferred stock warrants |
10.32 | ||||||
| Pro forma net tangible book value per share as of March 31, 2007, before this offering |
2.59 | ||||||
| Increase in pro forma net tangible book value per share attributable to new investors |
3.00 | ||||||
| Pro forma net tangible book value per share after this offering |
5.59 | ||||||
| Dilution in pro forma net tangible book value per share to new investors |
$ | 8.41 | |||||
A $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) the pro forma net tangible book value by $4.3 million, the pro forma net tangible book value per share after this offering by $0.28 per share and the dilution in pro forma net tangible book value per share to investors in this offering by $0.72 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their over-allotment option in full to purchase 697,500 additional shares of common stock in this offering, the pro forma as adjusted net tangible book value per share after the offering would be $5.92 per share, the increase in the pro forma net tangible book value per share to existing stockholders would be $3.33 per share and the dilution to new investors purchasing common stock in this offering would be $8.08 per share.
33
The following table shows on a pro forma basis at March 31, 2007, after giving effect to the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 8,339,296 shares of common stock upon the closing of this offering, the difference between the number of shares of common stock purchased from us, the total consideration paid to us and the average price paid per share by existing stockholders and by new public investors purchasing common stock in this offering:
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
10,611,282 | 70 | % | $ | 44,910,038 | 41 | % | $ | 4.23 | |||||
| New public investors |
4,650,000 | 30 | % | 65,100,000 | 59 | % | $ | 14.00 | ||||||
| Total |
15,261,282 | 100 | % | $ | 110,010,038 | 100 | % | $ | 7.21 | |||||
A $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) the total consideration paid by new investors by $4.7 million, or increase (decrease) the percent of total consideration paid by new investors by 2%, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
If the underwriters exercise their option in full, sales by us in this offering will reduce the percentage of shares held by existing stockholders to 66% and will increase the number of shares held by new investors to 5,347,500, or 34%.
This information is based on shares outstanding as of March 31, 2007 and excludes:
| • | 905,198 shares of common stock issuable upon the exercise of outstanding options to purchase common stock, at a weighted average exercise price of $1.52 per share; |
| • | 1,062,067 shares of common stock issuable upon the exercise of warrants for shares of Series A, Series B, Series C and Series D convertible preferred stock, on an as-converted basis at a weighted average exercise price of $5.20 per share; |
| • | 224,327 additional shares of common stock reserved for issuance under our 2003 Stock Option Plan; and |
| • | 2,000,000 additional shares of common stock for issuance under our 2007 Stock Plan, which becomes effective upon completion of this offering. |
To the extent these outstanding options or warrants are exercised, there will be further dilution to the new investors.
If all our outstanding options and warrants noted above had been exercised, the pro forma net tangible book value as of March 31, 2007 would have been $34,441,421, or $2.74 per share, and the pro forma net tangible book value after this offering would have been $92,234,421, or $5.35 per share, causing dilution to new investors of $8.65 per share. Additionally, assuming all outstanding options and warrants noted above had been exercised, the difference between the number of shares of common stock purchased from us, the total consideration paid to us, and the average price paid per share by existing stockholders and by new investors purchasing common stock in this offering would be as follows:
| Shares Purchased | Total Consideration | Average Price | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
12,578,547 | 73 | % | $ | 51,816,480 | 44 | % | $ | 4.12 | |||||
| New public investors |
4,650,000 | 27 | % | 65,100,000 | 56 | % | $ | 14.00 | ||||||
| Total |
17,228,547 | 100 | % | $ | 116,916,480 | 100 | % | $ | 6.79 | |||||
34
The following selected financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited financial statements and the related notes included elsewhere in this prospectus. The selected statement of operations data for the years ended December 31, 2004, 2005 and 2006 and selected balance sheet data as of December 31, 2005 and 2006 were derived from our audited financial statements that are included elsewhere in this prospectus. The selected statement of operations data for the three months ended March 31, 2006 and 2007 and for the period from December 10, 1996 (inception) through March 31, 2007 and selected balance sheet data as of March 31, 2007 were derived from our unaudited financial statements that are included elsewhere in this prospectus. The selected statement of operations data for the years ended December 31, 2002 and 2003 and selected balance sheet data as of December 31, 2002, 2003 and 2004 were derived from our financial statements audited by our former auditors and are not included in this prospectus. In the opinion of management, the unaudited financial statements were prepared on a basis consistent with our audited financial statements contained in this prospectus and include all adjustments necessary for the fair presentation of the financial information contained in those statements. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods, and the results for the three months ended March 31, 2007 are not necessarily indicative of results to be expected for the full year.
| Year ended December 31, | Three Months Ended March 31, |
Period
from March 31, 2007 |
||||||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||||||
| Grant revenue |
$ |
304,333 |
|
$ | 299,583 | $ | 208,252 | $ | 69,207 | $ | 94,850 | $ | — | $ | — | $ | 1,234,476 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Research and development |
207,298 | 380,771 | 1,419,293 | 2,966,991 | 4,974,380 | 1,100,125 | 1,479,340 | 11,802,694 | ||||||||||||||||||||||||
| General and administrative |
67,551 | 142,377 | 398,208 | 576,295 | 1,113,500 | 184,425 | 405,380 | 2,806,322 | ||||||||||||||||||||||||
| Sales and marketing |
— | — | — | 416,847 | 607,538 | 111,038 | 125,740 | 1,150,125 | ||||||||||||||||||||||||
| Total operating expenses |
274,849 | 523,148 | 1,817,501 | 3,960,133 | 6,695,418 | 1,395,588 | 2,010,460 | 15,759,141 | ||||||||||||||||||||||||
| Gain (loss) from operations |
29,484 | (223,565 | ) | (1,609,249 | ) | (3,890,926 | ) | (6,600,568 | ) | (1,395,588 | ) | (2,010,460 | ) | (14,524,665 | ) | |||||||||||||||||
| Interest income (expense), net |
(16,705 | ) | (6,863 | ) | 107,211 | 248,838 | 727,939 | 150,487 | 129,148 | 1,153,775 | ||||||||||||||||||||||
| Change in value of preferred stock warrants |
— | — | (254,089 | ) | (577,000 | ) | (290,925 | ) | (72,731 | ) | (3,681,413 | ) | (4,803,427 | ) | ||||||||||||||||||
| Net income (loss) |
$ | 12,779 | $ | (230,428 | ) | $ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,317,832 | ) | $ | (5,562,725 | ) | $ | (18,174,317 | ) | |||||||||
| Basic and diluted net income (loss) per share |
$0.01 | $(0.11 | ) | $(0.78 | ) | $(1.87 | ) | $(2.72 | ) | $(0.58 | ) | $(2.45 | ) | |||||||||||||||||||
| Weighted average number of shares outstanding—basic and diluted |
2,094,241 | 2,123,281 | 2,247,611 | 2,254,454 | 2,267,464 | 2,265,863 | 2,271,986 | |||||||||||||||||||||||||
| As of December 31, | As of March 31, |
|||||||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | ||||||||||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||||||||||
| Cash, cash equivalents and marketable securities |
$ | 5,841 | $ | 5,244,091 | $ | 10,583,799 | $ | 6,179,821 | $ | 13,469,135 | $ | 10,325,322 | ||||||||||||||||||||
| Working capital |
24,268 | 5,097,513 | 8,120,974 |
|
5,854,862 |
|
|
11,967,712 |
|
|
9,541,106 |
|
||||||||||||||||||||
| Total assets |
73,873 | 5,298,746 | 11,578,791 | 7,987,694 | 19,861,310 | 17,207,969 | ||||||||||||||||||||||||||
| Long-term debt, including current portion |
— | — | — | — | 1,621,898 | 1,556,241 | ||||||||||||||||||||||||||
| Convertible preferred stock |
— | 5,289,266 | 12,153,095 |
|
12,153,095 |
|
|
26,389,982 |
|
|
26,389,982 |
|
||||||||||||||||||||
| Total stockholders’ equity (deficit) |
(242,195 | ) | (173,258 | ) | (1,902,737 | ) | (6,066,589 | ) | (12,074,168 | ) | (17,559,966 | ) | ||||||||||||||||||||
35
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this prospectus. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. You should review the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis.
Overview
We are an orthopedic implants company focused on using our silicon nitride ceramic technologies to develop, manufacture and commercialize a broad range of advanced, high-performance spine and joint implants. We have developed a formulation of silicon nitride which we believe has the strength, toughness and wear resistance necessary to overcome the limitations of currently available orthopedic implants. Upon introduction to market, we believe our implants will represent the first commercial use of silicon nitride ceramics in orthopedic applications and will have the potential to provide an improved combination of characteristics, including greater strength and resistance to fracture, improved resistance to wear, greater ability to promote bone attachment and better compatibility with surgical and diagnostic imaging. Based on these potential advantages, we believe our silicon nitride product candidates may achieve better long-term clinical outcomes with enhanced durability, longevity, biocompatibility and patient fit. While we have not received regulatory clearance or approval for any of our product candidates that we intend to commercialize, in the second half of 2007 we expect to submit premarket notifications to the U.S. Food and Drug Administration, or the FDA, seeking regulatory clearance of our first commercial product candidate. Our goal is to establish our silicon nitride implants as new standards of care for the largest and fastest growing orthopedic implant markets: the spine, hip and knee markets.
Our lead product candidates are our Valeo™ family of spinal implants. Our Valeo spinal fixation implants are intended to restore and maintain the alignment of vertebrae in the cervical, or neck, region and lumbar, or lower back, region of the spine. The Valeo spinal fixation implants will feature silicon nitride ceramic spinal spacers for insertion between two vertebrae to help stabilize the spine, along with a metal cervical bone plate system and a metal pedicle screw system for supplemental fixation. We expect to launch the first of these product candidates by mid-2008, subject to clearance by the FDA. In 2006, we received clearance from the FDA for a silicon nitride ceramic spinal spacer, a device for insertion between two vertebrae to help stabilize the spine. We believe this is the first ceramic spinal spacer ever cleared by the FDA for human use. Although we do not plan to commercialize this spinal spacer, it will be the predicate device for our Valeo spinal spacers. We plan to introduce additional spinal spacers by the end of 2008, subject to regulatory clearance, including spacers that feature a bone-like structure with a solid, or cortical, load-bearing portion and a cancellous, or porous, structure that is intended to promote bone attachment for spinal fixation. In mid-2009, subject to regulatory approval, we plan to introduce cortico-cancellous spinal spacers with a surface coating designed to enhance bone attachment. Our Valeo family of spinal implant candidates also includes an all-ceramic, motion-preserving cervical disc, for which we anticipate commencing a clinical trial by mid-2009.
In addition, we are incorporating our silicon nitride ceramic technology into the development of our Infinia™ family of total hip and knee implants. We anticipate performing clinical trials for each of our Infinia product candidates. We believe that our Infinia Total Hip and Knee Implants, if approved or cleared by the FDA, may provide competitive advantages over currently marketed total hip and knee replacement implants. We are designing our Infinia Total Hip Implant to offer surgeons a range of sizes and design options comparable to implants with metal and plastic components but with improved wear resistance. We anticipate commencing a clinical trial for the first of these total hip implant candidates in 2009. We also are designing our Infinia Total Knee Implant and anticipate commencing a clinical trial for this product candidate in 2010. We believe that its
36
design will provide natural anatomic motion and offer lower wear and improved longevity compared to currently marketed knee replacement implants.
During the past two years, we have been designing and constructing our own manufacturing facility and developing processes that will provide us the ability to control the commercial-scale production of our silicon nitride ceramic implants from powder form to devices ready for sterilization and packaging. We are currently producing our lead ceramic spinal product candidates on a pilot scale in our manufacturing facility. We anticipate our facility will be fully operational for commercial-scale production by the end of 2007, which we believe would make us the only vertically integrated silicon nitride orthopedic implant manufacturer in the world.
We are a development stage company with a limited operating history and we currently have no product candidates cleared or approved for sale that we intend to commercialize. To date, our only significant revenue has been from research grants from the National Institutes of Health, or NIH, and with the exception of a small net income for the years ended December 31, 2002 and 1999, we have incurred net losses in each year since our inception. We expect our losses to continue and to increase as we build sales, marketing and distribution capabilities for our spine, hip and knee implant products, establish commercial-scale manufacturing operations, continue our research and development activities, and complete the regulatory clearance and approval process for our lead and pipeline product candidates, including the commencement of clinical trial activities for many of our pipeline product candidates. We plan to manufacture our own ceramic components while outsourcing other components. We have financed our operations to date primarily through private placements of our equity securities.
Revenue and Deferred Revenue
To date, we have not generated any revenue from the sale of any product. We do not expect to generate revenue from the sale of any product until the first half of 2008, subject to FDA clearance of our lead implant product candidates. We have received revenue from research grants from the NIH primarily related to development of ceramic implants. Revenue under grants is recognized when earned, and revenue is considered earned as the related qualified research and development expenses are incurred, up to the limit of the approved funding amounts.
Research and Development Expenses
Our research and development costs consist of engineering, product development, test-part manufacturing, testing, developing and validating our manufacturing process, and regulatory related costs. Research and development expenses also include employee compensation, employee and non-employee stock-based compensation, supplies and materials, consultant services, and travel and facilities expense related to research activities. From our inception through March 31, 2007, we have incurred approximately $11.8 million in research and development expenses.
We expect to incur increasing research and development expenses in future periods as we conduct more research and perform clinical trials for our Valeo Cervical Disc, Infinia Total Hip Implant and Infinia Total Knee Implant product candidates. We cannot predict our future research and development expenses with any degree of certainty.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation for executive, finance, and administrative personnel, including stock-based compensation, and facilities expenses related to general and administrative activities. Other significant expenses include travel expenses and professional fees for accounting and legal services. From our inception through March 31, 2007, we have incurred $2.8 million in general and administrative expenses. We expect our general and administrative expenses to increase during future periods to
37
support future growth and as a result of increased compensation costs related to additional personnel, as well as higher legal, accounting, insurance and other professional service costs relating to compliance with rules and regulations associated with being a publicly traded company.
Sales and Marketing Expenses
Our sales and marketing expenses consist primarily of compensation for sales and marketing personnel, including stock-based compensation, travel, consulting, marketing-related charges, and facilities expenses related to sales and marketing activities. From our inception through March 31, 2007, we have incurred $1.2 million in sales and marketing expenses related to the establishment and development of our sales and marketing infrastructure in anticipation of our 2008 product launches. We expect our sales and marketing expenses to increase due to the costs associated with the building of sales, marketing and distribution capabilities for our spine, hip and knee implant product candidates, including the amortization of our instrumentation sets to be used by surgeons to implant our product candidates.
Arrangements with Surgeon Advisors
We have entered into consulting and development agreements with some of our advisors, including some of our surgeon advisors. We have agreed to pay some of our surgeon advisors a portion of our net after-tax profits attributable to the sale of specific spine, hip and knee implant product candidates for which the surgeon advisor provided us with consulting and related services related to the conceptualization, development, testing, clearance, approval and/or related matters involving our implant product candidates. Because more than one of our surgeon advisors contribute to our development efforts, we are obligated to pay royalties to as many as five surgeon advisors in connection with some of our product candidates. These royalty payments will be recorded as cost of goods sold once we begin commercial sales of the relevant product candidate. Pursuant to these agreements, these surgeon advisors also have been granted options to purchase shares of our common stock. Generally, these consulting and development agreements, unless earlier terminated, continue until the later of (a) ten years from the date of the agreement and (b) the expiration of the patent rights relating to the product candidates covered by the agreement. We account for equity instruments issued to our surgeon advisors in accordance with the provisions of Emerging Issues Task Force, or EITF, No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, using a fair-value approach marked to market during the vesting period and record the related expense as research and development expense.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements and notes, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, convertible preferred stock, and stockholders’ equity (deficit) and the disclosure of contingent liabilities at the date of the financial statements, as well as the reported revenue and expenses during the reporting periods. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets, liabilities, convertible preferred stock, and stockholders’ equity (deficit) that are not readily apparent from other sources. We evaluate our estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 1 to our financial statements included elsewhere in this prospectus, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
38
Stock-Based Compensation
Prior to January 1, 2006, we accounted for stock-based employee compensation arrangements using the intrinsic value method in accordance with the recognition and measurement provisions of Accounting Principles Board Opinion, or APB, No. 25, Accounting for Stock Issued to Employees, and related interpretations, including the Financial Accounting Standards Board Interpretation, or FIN, No. 44, Accounting for Certain Transactions Involving Stock Compensation, an Interpretation of APB Opinion No. 25, as permitted by Statement of Financial Accounting Standards, or SFAS, No. 123, Accounting for Stock-Based Compensation. In accordance with APB No. 25, stock-based compensation was calculated using the intrinsic value method and represents the difference between the deemed per share market price of our common stock and the per share exercise price of the stock option. Based on this method, our compensation expense under APB No. 25 was zero.
Effective January 1, 2006, we adopted the provisions of SFAS No. 123R, Share-Based Payments. In March 2005, the Securities and Exchange Commission, or SEC, issued Staff Accounting Bulletin, or SAB, No. 107 relating to SFAS No. 123R. We have applied the provisions of SAB No. 107 in our adoption of SFAS No. 123R. Under SFAS No. 123R, stock-based awards, including stock options, are recorded at fair value as of the grant date and recognized to expense over the employee’s requisite service period (generally the vesting period), which we have elected to amortize on a straight-line basis. The pro forma disclosures previously permitted under SFAS No. 123 are no longer an alternative to financial statement recognition. We are no longer able to apply the minimum value method and instead must calculate the fair value of our employee stock options using an estimated volatility rate. We adopted the provisions of SFAS No. 123R using the prospective transition method. Under the prospective transition method, beginning January 1, 2006, compensation cost recognized includes: (a) compensation cost for all share-based payments granted prior to, but not yet vested as of December 31, 2005, based on the intrinsic value in accordance with the provisions of APB No. 25, and (b) compensation cost for all share-based payments granted subsequent to December 31, 2005, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123R. All awards granted, modified, or settled after the date of adoption are accounted for using the measurement, recognition, and attribution provisions of SFAS No. 123R.
Stock-based compensation expense, which is a non-cash charge, results from the issuance of options under SFAS No. 123R, based on the fair value of the stock options. During the year ended December 31, 2006 and the first three months of 2007, we granted options to employees and members of our board of directors to purchase a total of 165,054 shares of common stock, at an exercise price of $3.82 per share.
Our board of directors, with the assistance of management and LECG, LLC, or LECG, independent consultants, performed a contemporaneous fair value analysis for the value of our common stock as of October 31, 2006 and a retrospective fair value analyses for the valuation of our common stock as of December 31, 2005 and December 31, 2006. For grants made on dates for which there was no valuation performed by LECG to utilize in setting the exercise price of our common stock, and given the absence of an active market for our common stock, our board of directors determined the fair value of our common stock on the date of grant based on several factors, including:
| • | important developments in our operations; |
| • | equity market conditions affecting comparable public companies; |
| • | the likelihood of achieving a liquidity event for the shares of common stock, such as an initial public offering or an acquisition of the company, given prevailing market conditions; |
| • | the prices at which we issued preferred stock in February 2006 and the rights and privileges associated with those preferred stock issuances; and |
| • | the illiquidity of our common stock as a private company. |
In connection with the preparation of the financial statements included elsewhere in this prospectus, we utilized the results of LECG’s contemporaneous valuation as of October 31, 2006 and independent retrospective valuations as of December 31, 2005 and December 31, 2006 to determine the estimated fair value of our common
39
stock. The valuations used three valuation methods and weighted the results of these methods to determine the fair value of our total equity on the valuation dates. These three methods included a market approach using guideline public companies, a market approach using investment banker valuations and an income approach. The calculated total equity was then used to calculate the fair value per common share of our stock based on the option value method. The valuations also included a discount for lack of marketability given that there is no public market for our common stock.
The market approach using guideline public companies provides an estimate of fair value based on the value of similar public enterprises. These valuations included examination of the value of comparable companies in the orthopedic implant industry and other high growth med-tech companies. The market approach, using investment banker valuations, calculated the value of our equity based on estimated valuations provided by our investment bankers. The income approach utilizes the future cash flows expected to be generated and discounts the result to their present value using a rate of return that reflects the relative risk of the investment, as well as the time value of money.
The October 31, 2006 contemporaneous valuation and the December 31, 2005 and 2006 retrospective valuations weighted the calculated results of the above methods using a 75% weighting for the income approach, a 10% weighting for the market approach using guideline public companies and a 15% weighting for the market value using investment banker valuations. The weightings used in the overall valuation reflect management’s expectations based on anticipated cash flows, the likelihood of a near term public offering of our common stock and the ability to achieve liquidity in the public market. Because the income approach utilizes management’s perceived risks and opportunities that are specific to our company, this method received more weighting than the market approaches.
For periods between valuation dates, we reassessed the value of our common stock taking into account a number of factors including, among others, the proximity of the grant to the independent valuations and internal milestones that we achieved in the period between the two valuation dates.
Milestones affecting reassessed value between December 31, 2005 (our retrospective valuation) and February 12, 2006
In reassessing fair value per share between December 31, 2005, which, based on LECG’s retrospective valuation, was $2.75, and February 12, 2006, the date of our option grant at an estimated fair value per share of $3.82, we assigned an incremental value based on proximity of the grant to the retrospective valuation and the estimated value created by the interim milestones achieved during the period from December 31, 2005 and February 2006. Specifically, we considered the following additional milestones in connection with the grant:
| • | The anticipated FDA clearance of the Arx predicate device, our first ceramic spinal spacer, for which clearance was received in February 2006. |
| • | Receipt of a patent in February 2006 related to our Valeo Cervical Disc. |
| • | The sale in February 2006 of 8,400,000 shares of our Series C convertible preferred stock at $2.00 (or $7.64 per common share as converted) per share for gross proceeds of $16.8 million. |
| • | The fact that our common stock does not share the rights and preferences inherent in our preferred stock, including the liquidation preferences and dividend rights. |
As a result of these factors, we assessed the value of $3.82 assigned to our February 2006 option grants to be a reasonable estimate of the then fair value.
40
Milestones affecting reassessed value between October 31, 2006 (our contemporaneous valuation), December 31, 2006 (our retrospective valuation) and January 5, 2007
The contemporaneous valuation that we received as of October 2006 assessed the value of common shares at $3.78 per share and the December 31, 2006 retrospective valuation assessed fair value at $4.05 per share. The estimated fair value that we assigned to the common stock in December 2006 was $3.82 per share. No significant milestones occurred during the interim period between the date of the retrospective valuation (December 31, 2006) and the option grant in January 2007 that would significantly change the estimated fair value used for the January 2007 grant other than continued development of our product candidates.
Events occurring subsequent to January 5, 2007
In March 2007, our board of directors received a report from our legal counsel that served to validate and clarify our intellectual property position related to the patents that we have directed to our products and technologies. Based on the results of this report, we re-initiated discussions with investment bankers regarding our valuations and initial public offering prospects. Management believes that these events created a significant increase in the valuation of our company and our initial public offering prospects. As a result of these events, we also determined to seek additional financing which resulted in our issuance of Series D convertible preferred stock at $3.00 per share in April 2007. The Series D convertible preferred stock (as well as each of our other series of convertible preferred stock) included the following rights and privileges which are senior to our common stock:
| • | The Series D convertible preferred stock is entitled to receive noncumulative dividends in preference to any dividend on common stock if declared by our board of directors. |
| • | The Series D convertible preferred stock is entitled to a liquidation preference equal to the original issuance price of such stock, or $3.00 per share (or $11.46 per common share as converted). |
| • | The Series D convertible preferred stock is convertible into our common stock at the option of the preferred stockholder. The convertible preferred stock will be automatically converted into common stock at the completion of an initial public offering. |
| • | The holders of Series D convertible preferred stock are entitled to vote on all matters with the holders of the common stock based on the number of shares of common stock into which the preferred stock is convertible. |
| • | Since holders of the convertible preferred stock hold the majority of the voting shares in the aggregate, they substantially control decisions related to significant corporate matters requiring a vote of the shareholders. |
We granted no options between January 6, 2007 and May 3, 2007.
On May 4, 2007, we granted 101,141 options to employees and on May 6, 2007, we granted 27,489 options to certain of our directors, each option having an exercise price equal to $9.17 per share based on an additional contemporaneous valuation that we received from LECG. In connection with this valuation, we assessed the weightings placed on the valuation methods and increased the weightings of the market value using investment banker valuations based on the increased likelihood of a near-term initial public offering. Accordingly, this contemporaneous valuation weighted the calculated results of the three methods using a 50% weighting for the income approach, a 10% weighting for the market approach using guideline public companies and a 40% weighting for the market value using investment banker valuations. In this valuation, we also reduced the discount for lack of marketability due to the prospects for a near term liquidity event.
41
Based upon the assessments discussed above, we determined the estimated fair value of common stock related to options granted in 2006 and 2007 to be a reasonable estimate of the fair value. We took into account the factors identified above in determining the estimated fair value of the common stock as of each grant date. Information on employee and director stock options granted during 2006 and the first six months of 2007 is summarized as follows:
| Grant Date |
Number of Shares Granted |
Exercise Price per Share |
Reassessed Fair Value Per Share |
Intrinsic Value | |||||||
| 2/12/2006 |
50,589 | $ | 3.82 | $ | 3.82 | $ |
— | ||||
| 12/11/2006 |
97,449 | $ | 3.82 | $ | 3.82 | — | |||||
| 1/5/2007 |
17,016 | $ | 3.82 | $ | 4.05 | $ | 0.23 | ||||
| 5/4/2007 |
101,141 | $ | 9.17 | — | — | ||||||
| 5/6/2007 |
27,489 | $ | 9.17 | — | — | ||||||
Information on non-employee stock options granted in 2006 and the first six months of 2007 is summarized as follows:
| Grant Date |
Number of Shares Granted |
Exercise Price per Share |
Reassessed Fair Value Per Share |
Intrinsic Value | |||||||
| 2/12/2006 |
9,949 | $ | 3.82 | $ | 3.82 | $ | — | ||||
| 12/11/2006 |
38,490 | $ | 3.82 | $ | 3.82 | — | |||||
Based on an assumed initial public offering price of $14.00 per share, the intrinsic value of the options outstanding at March 31, 2007 was $11,296,256, of which $8,497,976 related to vested options and $2,798,280 related to unvested options.
The fair value of each employee option grant in the year ended December 31, 2006 and the three months ended March 31, 2006 and 2007 was estimated on the date of grant using the Black-Scholes valuation model with the following assumptions.
| Year ended December 31, 2006 |
Three months ended March 31, | |||||
| 2006 | 2007 | |||||
| (unaudited) | ||||||
| Weighted average risk-free interest rate |
4.53% | 4.58% | 4.67% | |||
| Weighted-average expected life (in years) |
6.25 | 6.25 | 5.00 | |||
| Expected dividend yield |
0% | 0% | 0% | |||
| Weighted average expected volatility |
91% | 101% | 81% | |||
| Weighted-average estimated fair value of employee options |
$2.98 |
$3.13 |
$2.79 | |||
Our computation of expected volatility for the year ended December 31, 2006 and the three months ended March 31, 2006 and 2007 is based on an average of the historical volatility of a peer-group of similar companies. Our computation of expected life utilizes the simplified method in accordance with SAB No. 107. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. We recognize stock-based compensation expense for the fair values of these awards on a straight-line basis over the requisite service period of each of these awards.
As of December 31, 2006 and March 31, 2007, total compensation related to unvested options not yet recognized in the financial statements was approximately $377,000 and $351,000, respectively, and the weighted average period over which it is expected to be recognized is approximately 3.66 and 3.45 years, respectively.
As a result of adopting SFAS No. 123R on January 1, 2006, the net loss for the year ended December 31, 2006 and three months ended March 31, 2006 and 2007 was higher by approximately $39,000, $5,000 and $28,000, respectively, than if we had continued to account for stock-based compensation under APB No. 25. Basic and diluted loss per share applicable to common stockholders for the periods presented would be the same as if we had continued to account for stock-based compensation under APB No. 25.
42
We account for equity instruments issued to non-employees in accordance with the provisions of Emerging Issues Task Force, or EITF, No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, using a fair value approach. The equity instruments, consisting of stock options and warrants granted to lenders and consultants, are valued using the Black-Scholes valuation model. The measurement of stock-based compensation is subject to periodic adjustments as the underlying equity instruments vest and are recognized as an expense over the term of the related financing or the period over which services are received.
We valued the non-employee stock options granted during the years ended December 31, 2004, 2005 and 2006 and the three month period ended March 31, 2007 using the Black-Scholes valuation model, using a volatility rate of between 83% and 101%, a remaining contractual life of between eight and ten years, an expected dividend yield of 0% and a risk-free interest rate ranging from 3.83% to 4.36%. We granted 18,326, 21,600 and 48,439 options to consultants for services in the years ended December 31, 2004, 2005 and 2006, respectively. No options were granted to consultants during the three months ended March 31, 2007. The exercise price of the consultant stock options ranges from $0.96 to $3.82 per share. The estimated fair value of options granted to consultants that vested during the years ended December 31, 2004, 2005 and 2006 and the three months ended March 31, 2006 and 2007 was $18,000, $44,000, $92,000, $23,000 and $49,000, respectively, and was charged to research and development expense. The amount of the stock compensation expense is subject to management’s estimate of the fair value of the underlying common stock absent a public market for our common stock.
Income Taxes
We account for income taxes under the liability method in accordance with the provision of SFAS No. 109, Accounting for Income Taxes (“SFAS 109”). SFAS 109 requires recognition of deferred taxes to provide for temporary differences between financial reporting and the tax basis of assets and liabilities. Deferred taxes are measured using enacted tax rates expected to be in effect in a year in which the basis difference is expected to reverse. We continue to record a valuation allowance for the full amount of deferred assets, which would otherwise be recorded for tax benefits relating to operating loss and tax credit carryforwards, since realization of such deferred tax assets cannot be determined to be more likely than not. Utilization of the net operating loss carryforwards may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of the net operating loss carryforwards before utilization.
In July 2006, the Financial Accounting Standards Board, or FASB, issued FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes (“FIN 48”). FIN 48 is an interpretation of FASB Statement No. 109, Accounting for Income Taxes. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of measurement and recognition in accounting for income taxes. In addition, FIN 48 provides guidance on derecognition, classification, interest and penalties, and accounting in interim periods and requires expanded disclosure with respect to the uncertainty in income taxes. We became subject to the provisions of FIN 48 as of January 1, 2007. We believe that our income tax filing positions and deductions will be sustained on audit and do not anticipate any adjustments that will result in a material change to our financial position. Therefore, no reserves for uncertain income tax positions have been recorded pursuant to FIN 48. In addition, we did not record a cumulative effect adjustment related to the adoption of FIN 48.
Our policy for recording interest and penalties associated with audits is to record such items as a component of income before taxes. Penalties and interest paid or received are recorded in interest expense or interest income, respectively. During the three months ended March 31, 2007, we did not record any interest income, interest expense, or penalties related to the settlement of audits for prior periods. Tax years 2003 through 2006 are subject to examination by the United States federal tax authorities.
43
Estimation of Fair Value of Warrants to Purchase Convertible Preferred Stock
In connection with our preferred stock offerings, the placement agent received warrants to purchase convertible preferred stock. These warrants are fully exercisable after one year from issuance and expire after seven years. The exercise price of these warrants is equal to 110% of the offering price of the underlying convertible preferred stock. On the closing of our initial public offering, these warrants will convert into warrants to purchase shares of common stock at the then applicable conversion rate for the related preferred stock (which will be 1-for-3.82 after giving effect to the reverse stock split which we effected on July 26, 2007).
We have accounted for these warrants under the provisions of Financial Accounting Standards Board Staff Position (“FSP”) No. 150-5, Issuer’s Accounting under Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that Are Redeemable, an interpretation of SFAS No. 150, Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity. Pursuant to FSP 150-5, freestanding warrants for shares that are either puttable or warrants for shares that are redeemable are classified as liabilities on the balance sheet at fair value. In connection with the grant of the warrants to purchase Series A and Series B convertible preferred stock in 2004 and Series C convertible preferred stock in 2006, the Company recorded the initial fair values of the warrants of $413,080, $159,831 and $928,625, respectively, as a preferred stock warrant liability. At the end of each reporting period, changes in fair value during the period are recorded as a component of other income or expense.
In order to estimate the liability associated with these warrants, management utilized an independent retrospective valuation performed by LECG as of each reporting date. The valuations used a market approach and an income approach to determine the fair value of our common stock into which the preferred stock is convertible on the valuation dates. The market approach bases fair value on what similar enterprises or comparable transactions indicate value to be. These valuations included examination of the value of comparable companies in the orthopedic implant industry and other high growth med-tech companies.
The fair value of these warrants was determined using the Black-Scholes valuation model using the following assumptions:
| Year ended December 31, | Three months ended March 31, | |||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||
| (unaudited) | ||||||||||
| Weighted-average risk-free interest rate |
3.85% | 4.36% | 4.70% | 4.70% |
4.55% | |||||
| Weighted-average remaining life (in years) |
6.24 | 5.24 | 4.85 | 4.85 |
4.60 | |||||
| Expected dividend yield |
0% | 0% | 0% | 0% |
0% | |||||
| Weighted-average expected volatility |
108% | 94% | 75% | 75% |
70% | |||||
For the years ended December 31, 2004, 2005 and 2006, and for the three month periods ended March 31, 2006 and 2007, we recorded approximately $254,089, $577,000, $290,925, $72,731, and $3,681,413, respectively, as other expense for the increase in fair value of all preferred stock warrants. We will continue to adjust the liabilities for changes in fair value until the earlier of the exercise of the warrants to purchase shares of convertible preferred stock or the completion of a liquidation event, including the completion of an initial public offering.
Upon the closing of this offering, all outstanding warrants to purchase shares of our preferred stock will become warrants to purchase shares of our common stock and, as a result, will no longer be subject to FSP 150-5. The then-current aggregate fair value of these warrants, after a final remeasurement of fair value, will be reclassified from liabilities to additional paid-in capital, a component of stockholders’ equity, and we will cease to record any related periodic fair value adjustments.
44
Results of Operations
Comparison of Three Months Ended March 31, 2006 and 2007
| Three months ended March 31, | Dollar |
% Change |
|||||||||||||
| 2006 | 2007 | ||||||||||||||
| (unaudited) | |||||||||||||||
| Grant revenue |
$ | — | $ | — | $ | — | * | ||||||||
| Operating expenses: |
|||||||||||||||
| Research and development |
1,100,125 | 1,479,340 | 379,215 | 34 | % | ||||||||||
| General and administrative |
184,425 | 405,380 | 220,955 | 120 | % | ||||||||||
| Sales and marketing |
111,038 | 125,740 | 14,702 | 13 | % | ||||||||||
| Total operating expenses |
1,395,588 | 2,010,460 | 614,872 | 44 | % | ||||||||||
| Loss from operations |
(1,395,588 | ) | (2,010,460 | ) | (614,872 | ) | 44 | % | |||||||
| Other income (expense): |
|||||||||||||||
| Interest income |
150,487 | 166,870 | 16,383 | 11 | % | ||||||||||
| Interest expense |
— | (37,722 | ) | (37,722 | ) | * | |||||||||
| Change in value of preferred stock warrant liability |
(72,731 | ) | (3,681,413 | ) | (3,608,682 | ) | 4,962 | % | |||||||
| Total other income (expense) |
77,756 | (3,552,265 | ) | (3,630,021 | ) | 4,668 | % | ||||||||
| Net loss |
$ | (1,317,832 | ) | $ | (5,562,725 | ) | $ | (4,244,893 | ) | 322 | % | ||||
| * | Not meaningful. |
Grant Revenue. We recorded no grant revenue in either period due to our internal product development projects in 2006 and 2007, which were not related to our existing grants. We were awarded an $800,000 NIH grant in late 2006, for which we expect to begin recognizing revenue in the second half of 2007 as we provide services under the related grant contract.
Research and Development Expenses. The increase in research and development expense in the three months ended March 31, 2007 compared to the same period in 2006 was due primarily to an increase in salaries and benefits of approximately $130,000, including the hiring of one additional person, an increase of approximately $180,000 from depreciation due to additional equipment in our manufacturing facility, and an increase in rent of $90,000 for our new manufacturing facility.
General and Administrative Expenses. The increase in general and administrative expenses for the three months ended March 31, 2007 compared to the same period in 2006 was due primarily to an additional $215,000 in legal fees incurred in the three months ended March 31, 2007 in connection with a review of our intellectual property position.
Sales and Marketing Expenses. The increase in sales and marketing expenses in the three months ended March 31, 2007 compared to the same period in 2006 was due primarily to consulting, marketing studies and trade show fees incurred during the three months ended March 31, 2007.
Interest Income. The increase in interest income in the three months ended March 31, 2007 compared to the same period in 2006 was due primarily to higher average interest rates in 2007.
Interest expense. Interest expense in the three months ended March 31, 2007 was related to our 42-month term loan which we entered into in January 2007 upon the conversion of our equipment financing arrangement, which allowed for advances to us for equipment purchased during 2006 and which we utilized during the second half of 2006.
45
Change in Value of Preferred Stock Warrant Liability. The increase in the change in value of preferred stock warrant liability for the three months ended March 31, 2007 compared to the same period in 2006 was due to valuation increases in our common stock into which the preferred stock is convertible as well as a greater number of warrants outstanding during the three months ended March 31, 2007 as a result of our Series C convertible preferred stock offering that closed on February 24, 2006. In April 2007, we issued additional warrants to purchase 253,290 shares of preferred stock to our placement agent and its designee in connection with our Series D convertible preferred stock offering. See Note 7 to our financial statements included elsewhere in this prospectus.
Comparison of Years Ended December 31, 2004, 2005 and 2006
| Years ended December 31, | Dollar |
% |
Years ended December 31, | Dollar Change |
% Change |
|||||||||||||||||||||||||
| 2004 | 2005 | 2005 | 2006 | |||||||||||||||||||||||||||
| Grant revenue |
$ | 208,252 | $ | 69,207 | $ | (139,045 | ) | (67 | )% | $ | 69,207 | $ | 94,850 | $ | 25,643 | 37 | % | |||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||
| Research and development |
1,419,293 | 2,966,991 | 1,547,698 | 109 | % | 2,966,991 | 4,974,380 | 2,007,389 | 68 | % | ||||||||||||||||||||
| General and administrative |
398,208 | 576,295 | 178,087 | 45 | % | 576,295 | 1,113,500 | 537,205 | 93 | % | ||||||||||||||||||||
| Sales and marketing |
— | 416,847 | 416,847 | * | 416,847 | 607,538 | 190,691 | 46 | % | |||||||||||||||||||||
| Total operating expenses |
1,817,501 | 3,960,133 | 2,142,632 | 118 | % | 3,960,133 | 6,695,418 | 2,735,285 | 69 | % | ||||||||||||||||||||
| Loss from operations |
(1,609,249 | ) | (3,890,926 | ) | (2,281,677 | ) | 142 | % | (3,890,926 | ) | (6,600,568 | ) | (2,709,642 | ) | 70 | % | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||||
| Interest income |
107,211 | 248,838 | 141,627 | 132 | % | 248,838 | 805,437 | 556,599 | 224 | % | ||||||||||||||||||||
| Interest expense |
— | — | — | * | — | (77,498 | ) | (77,498 | ) | * | ||||||||||||||||||||
| Change in value of preferred stock warrant liability |
|
(254,089 |
) |
(577,000 | ) |
|
(322,911 |
) |
127 | % | (577,000 | ) |
|
(290,925 |
) |
|
286,075 |
|
(50 | )% | ||||||||||
| Total other income |
|
(146,878 |
) |
(328,162 | ) |
|
(181,284 |
) |
123 | % | (328,162 | ) |
|
437,014 |
|
|
765,176 |
|
(233 | )% | ||||||||||
| Net loss |
$ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (2,462,961 | ) | 140 | % | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,944,466 | ) | 46 | % | ||||||||
| * | Not meaningful. |
Grant Revenue. The decreases in 2005 and 2006 grant revenue compared to the 2004 amount were due to our performing more NIH contract work in 2004 and our incurring fewer qualifying expenditures in 2005 and 2006 related to our NIH contracts as we refocused our efforts on internal product development projects.
Research and Development Expenses. The increase in research and development expenses in 2006 compared to 2005 was due primarily to an increase of approximately $800,000 for producing test parts and testing of our ceramics materials and producing prototypes and related instrumentation. We also experienced an increase of approximately $400,000 related to increases in our personnel costs, including the hiring of three additional employees, an increase of approximately $190,000 for additional rent and $340,000 of additional depreciation related to our new manufacturing facility and related equipment. The increase in research and development expenses in 2005 compared to 2004 was primarily attributable to an increase of $800,000 related to increases in personnel costs, including the hiring of nine additional employees, and $370,000 related to general lab supplies, product testing and validation costs. We also had an additional $140,000 of depreciation expenses in 2005 compared to 2004 due to the purchase of additional research equipment.
General and Administrative Expenses. The increases in general and administrative expenses in 2006 compared to 2005 was due primarily to $370,000 related to increases in personnel costs, including the hiring of three employees and $130,000 in legal fees in connection with a review of our intellectual property position. The
46
increase in general and administrative expenses in 2005 compared to 2004 was due primarily to an increase in legal costs of approximately $40,000 related to filing and protecting our patents, and an additional $70,000 in personnel costs related to the timing of employment commencement dates in 2004.
Sales and Marketing Expenses. In 2005, we commenced hiring of sales and marketing personnel to assist in the development of our product commercialization strategy. The increase in sales and marketing expense in 2006 compared with 2005 was due to an increase in personnel costs, including the hiring of one additional employee, as well as an increase in external market research and trade shows in preparation for our anticipated product candidate launches in 2008.
Interest Income. The increases in interest income in 2006 compared to 2005 and in 2005 compared to 2004 were due primarily to the timing of our receipt of the proceeds of our convertible preferred stock financings, which we invested in interest-bearing investments. These increases were also partially due to general increases in average interest rates in 2006 compared to 2005 and in 2005 compared to 2004.
Interest Expense. Interest expense in 2006 was for interest charges related to an equipment financing arrangement which we utilized during the second half of 2006 and under which we borrowed a total of $1.6 million. Interest on this financing arrangement was 10.25%. In January 2007, we converted the outstanding balance to a 42-month term loan bearing interest at the fixed rate of 9.09%.
Change in Value of Preferred Stock Warrant Liability. The increase in the change in value of preferred stock warrant liability in 2006 compared to 2005 and in 2005 compared to 2004 was due to valuation increases in our common stock as well as a greater number of warrants outstanding during 2006 compared to 2005 and in 2005 compared to 2004 as a result of our Series A preferred stock offering in January 2004, our Series B preferred stock offering in November 2004 and our Series C preferred stock offering in February 2006. See Note 7 to our financial statements included elsewhere in this prospectus.
Liquidity and Capital Resources
We have incurred a cumulative loss since our inception in December 1996 and as of March 31, 2007 we had a deficit accumulated during the development stage of $18.2 million. We have funded our operations to date principally from private placements of convertible preferred stock, raising net proceeds totaling $27.9 million through March 31, 2007. As of March 31, 2007, we had approximately $10.3 million in cash, cash equivalents and marketable securities included in current assets. We invest our available cash balances in bank deposits, money market funds, U.S. government securities, auction rate securities, and other investment grade debt securities that have strong credit ratings.
In April 2007, we issued 4,456,500 shares of our Series D convertible preferred stock at $3.00 per share, and received net proceeds of approximately $12.4 million. Dividends, liquidation preferences, conversion and voting rights of our Series D convertible preferred stock are substantially similar to those of Series A, B and C convertible preferred stock. In conjunction with this offering, the placement agent received warrants to purchase 253,290 shares of Series D convertible preferred stock at an exercise price of $3.30 per share. These warrants are fully exercisable at the earlier of one year after issuance or the completion of this offering and expire after seven years. The estimated fair value of these warrants at the time of issuance was $450,000. In connection with the offering of our Series D convertible preferred stock, we also paid our placement agent $758,870 as commission and $100,000 for expenses.
Net cash used in operating activities was $1.3 million, $3.5 million and $5.5 million for the years ended December 31, 2004, 2005 and 2006, respectively, and $761,000 and $2.4 million for the three months ended March 31, 2006 and 2007, respectively. The net cash used in each of these periods primarily reflects internal personnel costs associated with our research and development programs and infrastructure costs supporting our research and development activities. Included in net cash used in operating activities are non-cash charges related to revaluations of our preferred stock warrant liability, depreciation and amortization expense, and other net
47
changes in assets and liabilities affecting cash, including accounts payable and accrued liabilities that are primarily dependent upon the timing of our payments to our suppliers, vendors and employees.
Net cash provided by (used in) investing activities was ($9.3) million, $2.6 million and ($10.8) million for the years ended December 31, 2004, 2005 and 2006, respectively, and $(14.1) million, and $2.8 million for the three months ended March 31, 2006 and 2007, respectively. Net cash provided by (used in) investing activities in each of these periods reflects primarily the net purchases and maturities of marketable securities and purchases of property and equipment. Purchases of property and equipment increased in 2006 to $3.2 million compared to $854,000 in 2005 as a result of equipment purchasing and leasehold improvements associated with our new manufacturing facility.
Net cash provided by (used in) financing activities was $7.5 million, $17,000 and $16.8 million for the years ended December 31, 2004, 2005 and 2006, respectively, and $15.2 million and ($66,000) for the three months ended March 31, 2006 and 2007, respectively. Net cash provided by (used in) financing activities was attributable primarily to the issuance of shares of our Series A convertible preferred stock in January 2004, the issuance of shares of our Series B convertible preferred stock in November 2004, and the issuance of shares of our Series C convertible preferred stock in February 2006. In addition, we borrowed $1.6 million under our equipment financing arrangement during 2006 which was converted in early 2007 to a 42-month term loan. We made payments of $66,000 on the loan during the three months ended March 31, 2007.
We do not expect to generate any product revenue until the first half of 2008 at the earliest. We will not generate any domestic product revenue unless and until we obtain FDA clearance or approval to market our lead implant product candidates. We believe that our cash, cash equivalents and marketable securities, together with interest income we earn on these investments, will be sufficient to meet our anticipated cash requirements through the second quarter of 2008. We believe that the net proceeds from our initial public offering, together with our cash, cash equivalents and marketable securities, together with interest income we earn on these investments, will be sufficient to meet our anticipated cash requirements through the end of 2009. If our available cash, cash equivalents, marketable securities and net proceeds from our initial public offering are insufficient to satisfy our liquidity requirements, or if we develop additional product candidates or submit additional applications for clearance or approval of our product candidates, we may seek to sell additional equity or debt securities or arrange for a credit facility. The sale of additional equity and debt securities may result in additional dilution to our shareholders. If we raise additional funds through the issuance of equity or debt securities, these securities could have rights and preferences senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we are unable to obtain additional financing, we may be required to reduce the scope of, delay, or eliminate some or all of our planned research, product development and commercialization activities, which could materially harm our business.
Our forecast of the period of time through which our financial resources will be adequate to support our operations and the costs to complete development of product candidates are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the “Risk Factors” section of this prospectus. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
Because of the numerous risks and uncertainties associated with the development of medical devices, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to complete ongoing clinical trials and successfully deliver a commercial product to market. Our future funding requirements will depend on many factors, including but not limited to:
| • | the timing of regulatory clearances or approvals; |
| • | the scope, rate of progress and cost of our research and development activities; |
48
| • | the scope, rate of progress and cost of any clinical trials we are required to conduct, and the results of these clinical trials; |
| • | the cost and timing of establishing sales, marketing and distribution capabilities; |
| • | the rate of market acceptance of our product candidates; |
| • | the cost of filing and prosecuting patent applications and defending and enforcing our patents and other intellectual property rights; |
| • | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other third-party intellectual property rights; |
| • | the cost of defending other litigation or disputes with third parties; |
| • | the cost of establishing clinical and commercial supplies of our current pipeline of product candidates and any product candidates that we may develop; |
| • | the development of an efficient manufacturing process; |
| • | the effect of competing products and market developments; and |
| • | any revenue generated by sales of our future product candidates. |
Future capital requirements will also depend on the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.
Contractual Obligations
In May 2006, we entered into an equipment financing arrangement which allowed for advances to us for equipment purchased during 2006. These amounts are collateralized by certain of our qualifying manufacturing and lab equipment and $750,000 which is invested in an interest bearing escrow account and is reflected on the accompanying balance sheet as restricted cash. If the balance of our cash and marketable securities becomes equal to or less than the then remaining balance of the loan at any time during the term, the bank has the right to exercise a contingent pledge with respect to all remaining cash and marketable securities.
As of December 31, 2006, $1,621,898 had been advanced under this financing arrangement. In January 2007, this amount was refinanced into long-term debt with a fixed interest rate of 9.09% and with a 42-month term. Prior to May 23, 2007, the terms of this loan did not permit the transfer of more than 25% of the ownership interests in us. Effective May 23, 2007, the terms of this financing arrangement were amended to eliminate this transfer restriction upon the completion of our initial public offering.
We are committed to making future cash payments on operating leases. We have not guaranteed the debt of any other party. Future minimum payments under all noncancelable lease obligations and payments under our long-term debt agreement are as follows as of March 31, 2007:
| Year ended December 31, |
Operating Leases |
Long-term Debt |
|||||
| 2007 (remainder of year) |
$ | 339,875 | $ | 407,193 | |||
| 2008 |
|
463,392 |
542,922 | ||||
| 2009 |
|
400,991 |
542,922 | ||||
| 2010 |
|
263,154 |
316,705 | ||||
| 2011 |
|
91,605 |
— | ||||
| $ | 1,559,017 | 1,809,742 | |||||
| Less amounts representing interest |
(253,501 | ) | |||||
| $ | 1,556,241 | ||||||
49
The information above reflects only payment obligations that are fixed and determinable. Our commitments for operating leases primarily relate to the lease for our corporate headquarters and manufacturing facility in Salt Lake City, Utah.
In December 2006, we entered into an agreement to license patent rights directed to a manufacturing process for porous ceramic for use in our product candidates that incorporate our CSC technology. At the time this agreement was signed, we paid $50,000. During February 2007, we paid an additional $100,000 upon the transfer of technology to us. We are obligated to pay an additional $100,000 upon FDA clearance of the first product in the United States which utilizes the licensed technology. We are also obligated to pay future royalties on net sales of products which utilize this technology.
We have entered into consulting and development agreements with some of our advisors, including some of our surgeon advisors. We have agreed to pay some of our surgeon advisors a portion of our net after-tax profits attributable to the sale of specific spine, hip and knee implant product candidates for which the surgeon advisor provided us with consulting and related services related to the conceptualization, development, testing, clearance, approval and/or related matters involving our implant product candidates. Because more than one of our surgeon advisors contribute to our development efforts, we are obligated to pay royalties to as many as five surgeon advisors in connection with some of our product candidates. Pursuant to these agreements, these surgeon advisors also have been granted options to purchase shares of our common stock. Generally, these consulting and development agreements, unless earlier terminated, continue until the later of (a) ten years from the date of the agreement and (b) the expiration of the patent rights relating to the product candidates covered by the agreement.
We have executed agreements with some of our executive officers which, upon the occurrence of specified events related to a change of control, require payments to be made to the executives equal to two to three times his annual salary and accelerate the vesting of then outstanding stock options held by the executive.
Effective through February 2009, in the event of a future acquisition of our capital stock, merger, tender offer, recapitalization, or asset sale prior to our initial public offering resulting in a change in control, as defined in the engagement letter with the placement agent for our offering of Series C convertible preferred stock, the placement agent will have the right to receive up to $2.5 million.
Off-Balance Sheet Arrangements
Since inception, we have not engaged in any off-balance sheet activities, including the use of structured finance, special purpose entities or variable interest entities.
Quantitative and Qualitative Disclosures About Market Risk
Our exposure to market risk is confined to our cash, cash equivalents and marketable securities, all of which have maturities of less than one year. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and investments in investment grade marketable securities, including commercial paper, option rate preferred securities, money market funds and corporate debt securities and U.S. government securities. As of December 31, 2006 our marketable securities balance was approximately $11.8 million, which consisted of auction rate securities with rates that reset every 28 days. As of December 31, 2006, our long-term debt balance was approximately $1.6 million. The long-term debt bears interest at a fixed rate of 9.09% and has a term of 42 months. On a net basis, we believe that there is no material exposure to interest rate risk.
50
Overview
We are an orthopedic implants company focused on using our silicon nitride ceramic technologies to develop, manufacture and commercialize a broad range of advanced, high-performance spine and joint implants. We have developed a formulation of silicon nitride which we believe has the strength, toughness and wear resistance necessary to overcome the limitations of currently available orthopedic implants. Upon introduction to market, we believe our implants will represent the first commercial use of silicon nitride ceramics in orthopedic applications and will have the potential to provide an improved combination of characteristics, including greater strength and resistance to fracture, improved resistance to wear, greater ability to promote bone attachment and better compatibility with surgical and diagnostic imaging. Based on these potential advantages, we believe our silicon nitride product candidates may achieve better long-term clinical outcomes with enhanced durability, longevity, biocompatibility and patient fit. While we have not received regulatory clearance or approval for any of our product candidates that we intend to commercialize, in the second half of 2007 we expect to submit premarket notifications to the U.S. Food and Drug Administration, or the FDA, seeking regulatory clearance of our first commercial product candidate. Our goal is to establish our silicon nitride implants as new standards of care for the largest and fastest growing orthopedic implant markets: the spine, hip and knee markets.
Our lead product candidates are our Valeo™ family of spinal implants. Our Valeo spinal fixation implants are intended to restore and maintain the alignment of vertebrae in the cervical, or neck, region and lumbar, or lower back, region of the spine. The Valeo spinal fixation implants will feature silicon nitride ceramic spinal spacers for insertion between two vertebrae to help stabilize the spine, along with a metal cervical bone plate system and a metal pedicle screw system for supplemental fixation. We expect to launch the first of these product candidates by mid-2008, subject to clearance by the FDA. In 2006, we received clearance from the FDA for a silicon nitride ceramic spinal spacer, a device for insertion between two vertebrae to help stabilize the spine. We believe this is the first ceramic spinal spacer ever cleared by the FDA for human use. Although we do not plan to commercialize this spinal spacer, it will be the predicate device for our Valeo spinal spacers. We plan to introduce additional spinal spacers by the end of 2008, subject to regulatory clearance, including cortico-cancellous spacers that feature a bone-like structure with a solid, or cortical, load-bearing portion and a cancellous, or porous, structure that is intended to promote bone attachment for spinal fixation. In mid-2009, subject to regulatory approval, we plan to introduce cortico-cancellous spinal spacers with a surface coating designed to enhance bone attachment. Our Valeo family of spinal implant candidates also includes an all-ceramic, motion-preserving cervical disc, for which we anticipate commencing a clinical trial by mid-2009.
In addition, we are incorporating our silicon nitride ceramic technology into the development of our InfiniaTM family of total hip and knee implants. We anticipate performing clinical trials for each of our Infinia product candidates. We believe that our Infinia Total Hip and Knee Implants, if approved or cleared by the FDA, may provide competitive advantages over currently marketed total hip and knee replacement implants. We are designing our Infinia Total Hip Implant to offer surgeons a range of sizes and design options comparable to implants with metal and plastic components but with improved wear resistance. We anticipate commencing a clinical trial for the first of these total hip implant candidates in 2009. We also are designing our Infinia Total Knee Implant and anticipate commencing a clinical trail for this product candidate in 2010. We believe that its design will provide natural anatomic motion and offer lower wear and improved longevity compared to currently marketed knee replacement implants.
During the past two years, we have been designing and constructing our own manufacturing facility and developing processes that will provide us the ability to control the commercial-scale production of our silicon nitride ceramic implants from powder form to devices ready for sterilization and packaging. We are currently producing our lead ceramic spinal product candidates on a pilot scale in our manufacturing facility. We anticipate our facility will be fully operational for commercial-scale production by the end of 2007, which we believe would make us the only vertically integrated silicon nitride orthopedic implant manufacturer in the world.
51
Market Opportunity
According to the Millennium Research Group, in 2006, patients in the United States had approximately 1.5 million spinal fixation, hip replacement and knee replacement surgical procedures performed involving the use of implants, and this number is expected to grow primarily due to the rising incidence of arthritis. In 2005, an estimated 46 million U.S. adults suffered from doctor-diagnosed arthritis, and nearly two-thirds of those afflicted were younger than age 65. Osteo-arthritis, a condition involving the degeneration, or wearing away, of the cartilage at the end of bones, is a common form of arthritis, and often results in progressive joint disease and pain. The prescribed treatment for osteo-arthritis disorders depends on the severity and duration of the disorder and ranges from non-operative procedures including bed rest, medication, lifestyle modifications, exercise, physical therapy, chiropractic care and steroid injections, to surgical intervention including total joint replacement.
In cases where surgical intervention is prescribed, the use of implants has evolved into the standard of care in spine, hip and knee surgery. Surgeons replace affected joints with artificial implants, which currently are made from metal alloys, plastics such as polyethylene, allograft bone, or bone taken from a human cadaver donor, and ceramics. Implants such as hip and knee replacement implants, and motion-preserving disc implants, have components which move, or articulate, against each other and are known as articulating implants. Surgeons select the implants according to their patient’s weight, sex, age, activity level and other medical considerations. After surgery and rehabilitation, the patients usually experience less pain and swelling, and gain improved range of motion.
We believe that the market for implants used in spine, hip and knee surgical procedures will continue to grow because of the following market dynamics:
| • | Growth of the aging population. The population segment most likely to experience arthritis-related back and joint pain is expected to grow as a result of aging baby boomers, people born between 1946 and 1965, and increasing life expectancy. A majority of hip and knee replacement procedures performed in the United States are performed on the over-65 population, which is expected to increase from approximately 35 million in 2000 to approximately 71 million by 2030. |
| • | Changing lifestyle expectations. Middle-aged and older patients increasingly expect to enjoy active lifestyles, and consequently demand effective treatments for painful spine and joint conditions, including better performing and longer lasting implants. |
| • | Earlier surgical intervention. The clinical success of implant procedures, improved diagnostics, advances in minimally invasive surgical procedures, and better performing, longer lasting implants are expected to result in an increase in surgical treatment of patients at a younger age. |
| • | Rising number of revision surgeries. Premature failures of currently available implants, due in part to the limitations of low wear resistance materials, have resulted in a rapid rise in revision surgeries to replace worn or defective implants. In 2006, approximately 49,700 revision hip surgeries and 48,000 revision knee surgeries were performed in the United States. An estimated 73,400 revision hip surgeries and 87,400 revision knee surgeries will be performed in the United States in 2011. |
| • | Introduction of new technologies. Newer implants with improved function, such as articulating disc implants and other motion-preserving implant systems, are expected to drive additional growth of the spinal market. In addition, longer lasting implants are expected to drive growth in the hip and knee market by being indicated at an earlier stage for patients with degenerative joint disease. |
| • | Market expansion into new geographic areas. As implant procedures are introduced to and become more widely accepted in underserved countries such as China and India, it is anticipated that demand for implants will increase. |
52
Spine Implant Market
The spine market is the fastest growing market for orthopedic implants, accounting for $3.3 billion in sales in the United States in 2006, and is projected to grow at an average annual rate of 12.0% through 2011 to approximately $5.9 billion. Spinal fixation surgeries currently represent the vast majority of procedures in this market. Approximately 500,000 spinal fixation surgery procedures were performed in the United States in 2006, accounting for approximately $3.2 billion of the total $3.3 billion in U.S. spine market sales.
Limitations of Current Spinal Implants
Spinal fixation is the current standard of care for the surgical treatment of disc herniation for patients who have chronic pain and who have or are likely to develop associated spinal instability. Disc herniation is a condition where the disc bulges from between two vertebrae and impinges on nerves causing pain. Spinal fixation procedures aim to relieve the impingement on the nerves by removing the portion of the disc and/or bone responsible for compressing the neural structures and destabilizing the spine. The excised disc or bone is replaced with one or more intervertebral implants, or spacers, placed between the adjacent vertebrae. To provide initial support and long-term stabilization, surgeons commonly use supplemental fixation implants to immobilize the treated area of the spine. These supplemental fixation implants consist of a pedicle screw system for the lumbar area of the spine, and a bone plate system for the cervical area of the spine. To enhance bone attachment, surgeons often pack pulverized bone harvested from the patient, known as autograft bone, in and around fixation implants. Autograft bone is rich in natural bone morphogenic proteins, or BMPs, which promote growth of new bone tissue into and around spinal implants to fuse the vertebrae. As an alternative, synthetic BMPs are also often used to promote bone growth and achieve fusion more quickly.
Currently marketed spine implants have significant performance limitations due to the materials from which they are made. Spine implants are manufactured using allograft, or cadaver bone; metals, such as titanium; or plastics, such as polyetheretherketone, or PEEK. While all of these materials enable manufacturers to produce load-bearing spacers, none of the currently available spinal spacers possess all of the important characteristics desired by surgeons. Drawbacks include:
| • | Limited availability and inconsistent quality of allograft bone. Allograft bone is in short supply and is subject to inconsistent quality and size, often requiring surgeons to make compromises on fit while operating on patients. Patients also face a relatively remote but finite risk of disease transmission and immune response when introducing allograft bone into the body. |
| • | Current materials require supplemental bone fusion promoters. Current spacer materials are bio-inert and require growth factors such as synthetic BMPs to promote bone attachment. However, with cost-containment initiatives from hospitals and public and private payors alike, separate reimbursement for the use of BMPs in spinal fixation procedures have come under increasing cost/benefit scrutiny. BMPs are also known to provoke inflammation in the cervical spine and to form abnormal bone growth in the lumbar spine, which may impinge on neural structures. Alternatively, surgeons can use autograft bone which requires secondary surgery, resulting in increased pain, a longer recovery period, higher costs and greater risk of infection. |
| • | Currently available spinal implant materials lack optimal imaging-compatible characteristics. Surgeons use X-ray imaging and magnetic resonance imaging, or MRI, during spinal fixation procedures to assist in the proper placement of implants, as well as to assess the quality of post-operative bone fusion. Traditional metal alloy materials restrict the ability of physicians to detect the extent and quality of bone attachment and ingrowth due to their X-ray density or magnetic nature. PEEK products are hard to detect using X-ray, even with the embedded markers that surgeons use to enable them to place the implants during surgery and confirm their location and the quality of attachment to bone after surgery. |
Although spinal fixation procedures may address symptoms in the short term, they prevent natural movement among vertebrae, which can result in a reduction in a patient’s range of natural motion and accelerated degeneration of healthy discs at levels above and below the fixed vertebrae. Therefore, many orthopedic implant
53
companies are pursuing the development of a new generation of disc replacement implants designed to restore natural motion between vertebrae. Similar to the evolution of articulating implants for hips and knees, motion-preserving disc implants are emerging as a promising alternative to spinal fixation. To date, the only motion-preserving disc replacement implants approved for marketing in the United States are metal-on-polyethylene implants, which have a risk of slippage or dislocation due to a three-piece construction. Although the clinical experience of these implants is recent and longer-term outcomes are not established, they may have limitations due to unfavorable imaging and wear characteristics commonly associated with metal-on-polyethylene articulating implants.
Hip and Knee Implant Market
Orthopedic implants used in hip and knee replacement surgeries generated approximately $5.6 billion in sales in 2006 in the United States, and such sales are projected to increase at an average annual rate of 9.4% through 2011 to approximately $8.8 billion. Approximately one million primary hip and knee replacement procedures were performed in the United States in 2006.
Limitations of Current Hip and Knee Implants
Total hip replacement involves removing the diseased joint and replacing it with an artificial hip implant. This procedure entails the removal of the head, or the upper end of the thigh bone, known as the femur, and replacing it with an artificial femoral head, consisting of a ball mounted on an artificial stem which is inserted into the femur. The artificial femoral head articulates against an artificial socket, called an acetabular liner, which is placed inside an acetabular cup affixed into the pelvic bone. The femoral head and acetabular liner are often referred to as bearings.
Total knee replacement also involves removing the diseased joint and replacing it with an artificial knee implant. This procedure entails removal of the lower end of the femur, known as the condyle, removal of the upper end of the major lower leg bone, or tibia, and the removal of the knee cap, or patella. Following removal of the diseased tissue, an artificial knee implant is inserted, consisting of four main components: a femoral condyle, or a specially shaped bearing that is affixed to the lower end of the femur; a tibial tray that is affixed to the upper end of the tibia; a tibial insert that is rigidly fixed to the tibial tray and serves as the surface against which the femoral bearing moves; and a patella.
Because articulating implants have components that move against each other, causing friction, wear debris has been a clinical problem experienced in the hip and knee implant market. With conventional articulating implants, such as a hip replacement implant with a metal femoral head articulating against a polyethylene acetabular liner, friction can degrade the liner, causing small polyethylene wear particles to break off in the body. The human immune system rejects this foreign debris, attacking it much like it would attack an infection. Because wear debris typically settles around the site of the implant, the immune system also attacks and degrades the surrounding bone tissue, which is known as osteolysis. As a patient loses bone tissue in his or her hip, the implant can become loose and unable to function.
Orthopedic surgeons identify osteolysis as a leading cause of joint implant failure, resulting in the need for revision procedures to replace the failed implant. Ever since the connection between polyethylene particles and implant failures was established over two decades ago, articulating joint components made with alternative materials, including cross-linked polyethylene, metals and ceramics, have been used to reduce wear. Currently available implants for total hip and knee replacements are primarily differentiated by the materials used for the alternate bearing surfaces of the implants, the most common of which are metal-on-cross-linked polyethylene, metal-on-metal, and ceramic-on-ceramic. While all of these alternative materials have resulted in improved wear resistance, none of them possess all of the important characteristics desired by surgeons. Drawbacks include:
| • | Risk of premature implant failure with cross-linked polyethylene. Despite its greater resistance to wear, cross-linked polyethylene is more brittle than traditional polyethylene and consequently is prone to |
54
| fatigue failure. In addition, cross-linked polyethylene wear particles can generate a stronger adverse biologic response, resulting in bone loss and premature implant failure, the very problem that cross-linked polyethylene was designed to address. |
| • | Design and size limitations of implants with cross-linked polyethylene. Surgeons prefer the use of larger diameter femoral heads because they are less likely to dislocate from the acetabular liners. However, higher wear is observed for larger sized cross-linked polyethylene liners, leading to greater wear debris. Consequently, some surgeons avoid the use of large sized cross-linked polyethylene components. |
| • | High metal ion concentrations with metal-on-metal bearings. Metal-on-metal bearings generate fine metal particles or metal ions which are absorbed in the body. These particles are excreted from the body through the kidneys. Increasingly, surgeons are voicing concerns about the potential toxic effects of such high metal concentrations and the burden on the kidneys. Some patients also exhibit a hypersensitivity or allergic response to metals in their body. |
| • | Design and size limitations of implants with currently available ceramic bearings. Currently marketed ceramic implants are limited by the strength and fracture resistance of currently available ceramic materials. Current ceramic acetabular cups, for example, require a metal shell to contain and reinforce the ceramic liner. This two-piece design limits the size of the femoral head that may be used because of cumulative thickness of the multiple pieces in a restricted joint space. However, physicians prefer the use of larger heads to minimize the risk of hip dislocation. In addition, ceramic femoral heads are limited in the depths to which they can be seated on the femoral stem, thereby restricting the ability to restore proper leg length. Significant leg length discrepancy results in a limp, which is the primary cause for malpractice lawsuits related to hip replacement surgeries. |
We believe the rising market demand for orthopedic implants, coupled with the significant drawbacks associated with existing materials used for current implants, creates a substantial market opportunity for implants made with higher performing materials.
Our Solution
We believe our silicon nitride ceramic technologies, MC2 and CSC, will overcome many of the limitations associated with currently available implant materials by providing an improved combination of characteristics, including:
| • | greater strength and resistance to fracture than currently marketed ceramic implants; |
| • | improved resistance to wear compared to implants made of plastics and metals; |
| • | greater ability to promote bone attachment than traditional plastic and metal implants such as PEEK and titanium; and |
| • | better compatibility with surgical and diagnostic imaging techniques. |
We believe that the anticipated greater strength and fracture resistance of our silicon nitride product candidates will allow us to offer a wider range of design and size options and lower risk of fracture compared to currently marketed implants made of ceramic materials. We further believe that the anticipated improved wear resistance and the biocompatibility over the life of our silicon nitride implant candidates will reduce the risk of osteolysis and allergic response to metal wear particles. Based on these potential advantages, we believe that our silicon nitride product candidates may achieve better long-term clinical outcomes with a combination of improved durability, longevity, biocompatibility and patient fit.
Micro-Composite Ceramic, or MC2. We refer to our formulation of silicon nitride as MC2, or Micro-Composite Ceramic. We expect that all of our ceramic product candidates will be made using our MC2 silicon nitride. Since our inception we have focused on the development of a uniformly dense, micro-particle formulation of silicon nitride ceramic that has the strength, toughness and wear resistance necessary to overcome
55
the limitations of currently available orthopedic implants. This ceramic is made from silicon nitride formulated with dopants such as yttria and alumina. We believe we are the first company to engage in the development of ceramic-based spine and joint implants made from silicon nitride, which in the past has been used in mission-critical aerospace and other applications requiring high-strength and low-friction of moving parts. For the demanding applications represented by human implants, we produce a biocompatible silicon nitride ceramic of precise specifications with the high strength and toughness necessary to achieve the required fracture resistance and reliability. We believe that we have developed significant know-how related to the manufacture of spine and joint implants made from silicon nitride. We also have an issued patent and pending patent applications directed to certain articulating implants with silicon nitride components.
Cortico-cancellous Structured Ceramic, or CS C. We also are developing implants made with our MC2 silicon nitride that mimic the structure of natural bone by incorporating both a dense load-bearing component and a porous component, coupled with a surface coating, intended to promote bone attachment. We call our ceramic implants based on this technology CSC, or Cortico-cancellous Structured Ceramic, implants. We are developing our CSC implants for applications where the promotion of bone attachment is important for successful implant fixation. We have been issued two U.S. patents directed to our implants that will have both a dense load-bearing, or cortical, component and a porous, or cancellous, component, together with a surface coating. We also have pending patent applications directed to our cortico-cancellous structured implants using our CSC technology. In addition, we have exclusively licensed three U.S. patents and foreign counterparts, together with related know-how, directed to manufacturing processes for the production of porous ceramics for use in our orthopedic implants.
Our Strategy
Our goal is to become a leading orthopedic company offering advanced silicon nitride ceramic implants for a broad range of orthopedic indications. We intend to use our ceramic technologies to develop implants that have performance advantages compared to existing implants. We believe that the combined benefits of our MC2 and CSC technologies will give our product candidates the potential to become a new standard of care for spine, hip and knee procedures.
Key elements of our strategy to achieve this objective include the following:
| • | Launch near-term product candidates that address substantial market opportunities and build market awareness. We intend to pursue the FDA’s 510(k) pathway for clearance of our lead product candidates targeting spinal fixation, adding stepwise improvements to previously cleared products to help expedite the FDA review process. Subject to FDA clearance, we expect to introduce three of our lead spinal fixation product candidates, the Valeo Cortical Ceramic Spacers, the Valeo Cervical Plate System and the Valeo Pedicle Screw System by mid-2008. Thereafter, we plan to introduce our Valeo Cortico-Cancellous Spinal Spacers by the end of 2008, followed by our Valeo Coated Cortico-Cancellous Spinal Spacers by mid-2009. We expect that the introduction of these product candidates will accelerate physician awareness and acceptance of our ceramic-based implants and therefore help to facilitate market penetration of our pipeline product candidates. |
| • | Build a broad portfolio of ceramic implants targeting expanded indications and additional surgical procedures. We are continuing to develop our product candidate portfolio to introduce future generations of implant products and expand the range of surgical indications and procedures that our implants address. These next generation product candidates will enable us to offer the broad suite of implants sought by orthopedic surgeons and distributors, including total disc replacements and total hip and total knee implant systems. We believe that the product candidates we are developing will be longer lasting and can be used for earlier intervention, resulting in better patient care. |
| • | Utilize the expertise of our surgeon advisors to design physician-preferred product features. Our surgeon advisors participate throughout our product development process for both our spine and joint reconstructive product candidates. Our surgeon advisors include leading orthopedic surgeons in the |
56
| United States, such as Aaron A. Hofmann, M.D., the designer of the Natural KneeTM, a widely used total knee implant. We work closely with our surgeon advisors in the design of our implants and expect to benefit from their strong networks of national and international surgeon relationships to facilitate awareness of our product candidates. |
| • | Establish a hybrid sales organization utilizing experienced, independent sales agencies and a direct sales force. We intend to partner with independent sales agencies to leverage their experience and strong surgeon relationships in both the spine and joint reconstructive markets to help us penetrate both U.S. and international markets. In the United States, we plan to establish a direct sales force to complement our independent sales agents in selected markets. |
| • | Selectively establish collaborations for our implants with leading orthopedic companies. We intend to develop collaborations in markets outside of the United States where we believe that having a large, well-established partner will enable us more efficiently to gain access to those markets. In addition, we may establish collaborations in the United States in instances when access to a larger sales and marketing organization may help to expand the market or accelerate penetration for selected product candidates. |
Our Product Candidates
The table below identifies our products under development, lists their regulatory status, and indicates the anticipated launch dates of our lead spinal fixation product candidates and the anticipated start of clinical trials of product candidates in our development pipeline.
57
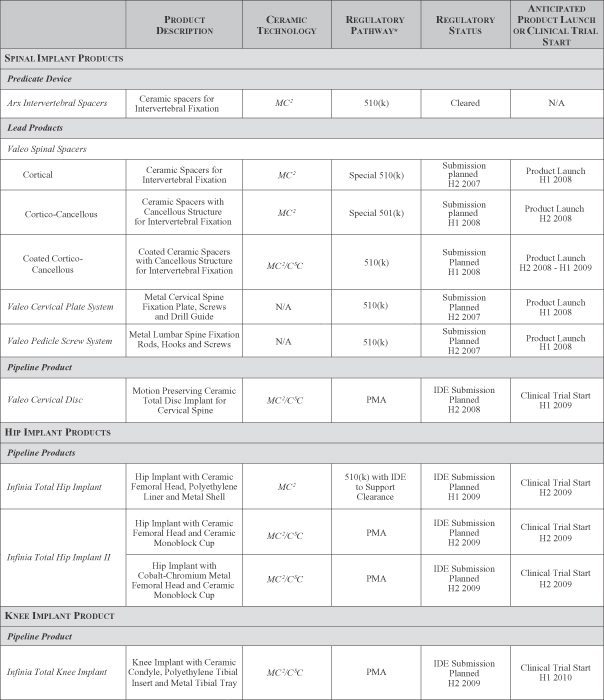
| * | Anticipated regulatory pathways for future submissions to the FDA of applications for product candidates. |
Our planned strategy for clearance of our Valeo Cortical Spinal Spacers described in the foregoing table will be to submit two successive Special 510(k) notifications for clearance of two different aspects of this product candidate. We expect these Special 510(k) clearances will serve as the basis for clearance of the next spinal spacers product candidate we plan to launch.
58
Our Spinal Implant Products
We have designed our lead product candidates in our Valeo family of spinal implants as a comprehensive solution for surgical procedures for spinal fixation. These products include spinal spacers, a cervical bone plate system, a pedicle screw system, and a set of surgical instruments that facilitate the placement of our implants in the body. We are also developing an all-ceramic motion-preserving cervical disc.
Valeo Spinal Spacers
We have designed our Valeo family of spinal spacers, using silicon nitride ceramic, as intervertebral fixation implants for stabilizing the spine by replacing a portion of a vertebra that has collapsed, been damaged, or becomes unstable due to disease or trauma. Intervertebral implants were estimated to comprise an annual U.S. market of approximately $805 million in 2006. We believe that each of our Valeo Spinal Spacers, if cleared by the FDA, will have competitive advantages compared to existing spinal implants made from allograft bone, PEEK or metals, and the ability to permit X-ray and MRI imaging during and after the surgical procedure. We developed and received FDA clearance for our Arx Intervertebral Spacers made from MC2 silicon nitride, which will serve as the predicate device for the 501(k) premarket notification for our Valeo Cortical Spinal Spacers product candidate.
Cortical. We are conducting tests on our Valeo Cortical Spinal Spacers made of silicon nitride ceramic in anticipation of submitting two successive Special 510(k)s in the second half of 2007. We anticipate launching our Valeo Cortical Spinal Spacers by mid-2008.
Cortico-Cancellous. We have designed our Valeo Cortico-Cancellous Spinal Spacers to be identical to our Valeo Cortical Spinal Spacers except that this implant, in addition to its cortical structure, also will include a cancellous, or porous, structure that will facilitate bone attachment to the cancellous portion of the implant. We are conducting tests on our Valeo Cortico-Cancellous Spinal Spacers in anticipation of submitting a Special 510(k) to the FDA by mid-2008 and we anticipate launching the Valeo Cortico-Cancellous Spinal Spacers by the end of 2008.
Coated Cortico-Cancellous. We have designed our Valeo Coated Cortico-Cancellous Spinal Spacers to be identical to our Valeo Cortico-Cancellous Spinal Spacers except that this product candidate also will include a surface coating made of hydroxy-apatite designed to enable natural bone to more effectively attach to the cancellous portion of the implant. We are conducting tests on our Valeo Coated Cortico-Cancellous Spinal Spacers in anticipation of submitting a 510(k) premarket notification to the FDA by mid-2008, and we anticipate launching the Valeo Coated Cortico-Cancellous Spinal Spacers in mid-2009.
Valeo Cervical Plate System
We are developing our Valeo Cervical Plate System as a titanium alloy supplemental fixation implant to be used in conjunction with our Valeo Spinal Spacers. Currently available bone plate systems accounted for an estimated $428 million in U.S. sales in 2006. We consulted extensively with our spine surgeon advisors in the design of the Valeo cervical plate product and related surgical instruments in order to incorporate features aimed at making cervical fixation procedures more efficient, simpler and more consistent. Our design and instruments combine special features to enable surgeons, in a single step, to hold the cervical plate in place, ensure proper angling and insertion of the screws into the vertebrae, and achieve a consistent supplemental fixation outcome. We also believe that our Valeo Cervical Plate System product candidate, because of its design features, may be an attractive option for use with other spacers for cervical spine fixation.
We have finalized the design of our Valeo Cervical Plate System. We anticipate completing product testing and verification and submitting a 510(k) premarket notification in the second half of 2007. We anticipate launching this product by mid-2008.
59
Valeo Pedicle Screw System
We are developing our Valeo Pedicle Screw System as a titanium alloy, low profile, and modular implant system to be used in conjunction with our Valeo Spinal Spacers for supplemental fixation of the lumbar spine. Pedicle screw systems accounted for an estimated $1.2 billion in U.S. sales in 2006. We consulted extensively with our spine surgeon advisors in the design of the Valeo Pedicle Screw System in order to incorporate features allowing surgeons greater flexibility in the positioning of screws and rods and selection of rod diameters during surgery. We have designed modularity in the system components to permit such flexibility, which we believe will provide better clinical outcomes. We also believe that our Valeo Pedicle Screw System product candidate, because of its design features, may be an attractive option for use with other spacers for lumbar spine fixation.
We have finalized the design of our Valeo Pedicle Screw System. We anticipate completing the product testing and verification and submitting a 510(k) premarket notification in the second half of 2007. We anticipate launching this product by mid-2008.
Valeo Cervical Disc
We are developing our Valeo Cervical Disc, using both our MC2 and CSC technologies, as a silicon nitride ceramic implant to meet the unmet market need for a disc replacement implant that will restore natural motion and provide uncompromised wear resistance and favorable imaging characteristics in the cervical spine. This product candidate is aimed at the cervical disc market which is estimated to generate in excess of $450 million in U.S. sales by 2011. We believe to date, that two companies have introduced lumbar discs which seek to mimic the natural biomechanics of the spine, and numerous other orthopedic implant companies are pursuing the development of disc implants to restore natural spinal motion. However, to our knowledge, most of these companies are using traditional metal-on-polyethylene bearings or metal-on-metal bearings, both of which are known to produce wear debris with less than optimal long-term outcomes. To our knowledge, a small number of companies are pursuing the development of disc implants with ceramic bearing surfaces, but we understand these bearing surfaces are embedded in metal backings which may interfere with imaging. We believe our Valeo Cervical Disc, if approved by the FDA, will represent a significant advance over currently available disc implants by mimicking the natural biomechanics of the spine, eliminating plastic and metal wear particles, promoting secure attachment to adjacent vertebrae, and allowing improved X-ray and MRI imaging.
We are finalizing the design of our Valeo Cervical Disc. We anticipate submitting an IDE, or investigational device exemption, application to the FDA in the second half of 2008, and beginning clinical trials of this implant in the first half of 2009. We are also exploring the possibility of developing a similar product for use in the lumbar spine.
Our Hip Implant Products
Infinia Total Hip Implant
We are developing our Infinia Total Hip Implant for patients undergoing total hip replacement surgery for the treatment of degenerative joint disease. This product candidate targets the market for total hip implants, estimated at $2.4 billion in the United States in 2006. In our first hip replacement implant, we will use silicon nitride ceramic for the femoral head component of this implant. The counter-bearing, or mating component, of the hip implant, will be a polyethylene liner, fixed into a metal acetabular cup, using industry-recognized designs and materials. We anticipate that our Infinia Total Hip Implant, if cleared by the FDA based on clinical trial results, will provide the following significant competitive advantages over traditional total hip replacement implants presently on the market:
| • | Our femoral head will use silicon nitride ceramic material that significantly reduces polyethylene wear debris which is believed to be the primary reason for implant failures in implants using plastic bearings. We believe this reduction in wear debris should significantly improve the performance and longevity of our implants; and |
60
| • | Our use of silicon nitride ceramic will enable us to offer femoral heads with sizing options comparable to metal femoral heads and substantially greater than currently marketed ceramic heads. This will reduce the dislocation risk associated with smaller femoral heads. In addition, our silicon nitride ceramic will enable us to offer offset sizing options comparable to metal femoral heads which will minimize, if not entirely eliminate, the need for surgeons to settle for less than optimal leg length results. We also believe that we will be able to offer heads with substantially greater strength than currently marketed ceramic heads. |
We are finalizing the design of our Infinia Total Hip Implant. We anticipate submitting a 510(k) premarket notification and an IDE application to perform clinical trials to support our 510(k) premarket notification to the FDA in the first half of 2009, and beginning clinical trials of this implant in the second half of 2009.
Infinia Total Hip Implant II
We are developing our second generation Infinia Total Hip Implant II featuring our Infinia monoblock cup, an industry-first, one-piece, fully ceramic acetabular cup, our large diameter Infinia ceramic and metal femoral heads, and our Infinia femoral stem. The Infinia monoblock cup will be made from our MC2 silicon nitride ceramic and will incorporate a smooth bearing surface on the inside of the cup integrated with a bone attachment surface incorporating our CSC technology on the outside of the cup that comes into contact with a patient’s pelvis. The strength of silicon nitride ceramic allows us to design the Infinia monoblock cup as a one-piece component without the need for a separate liner, which will allow the use of larger femoral head sizes. Larger diameter femoral heads are known to have a lower incidence of dislocation.
The femoral head of the implant will be a large-diameter head offered in two versions, one made of silicon nitride and the other of cobalt-chromium. The femoral head will be used with a metal stem inserted into the femur. In contrast to currently marketed ceramic femoral heads, we are designing our MC2 femoral head to offer surgeons a range of size and design options comparable to those available in metal femoral heads.
We are currently designing our Infinia monoblock cup, femoral head and femoral stem components. We have also begun testing the various silicon nitride components of our Infinia Total Hip Implant II. We anticipate that the femoral stem utilized in our first generation Infinia Total Hip Implant will be incorporated in our Infinia Total Hip Implant II. We anticipate submitting an IDE application for our Infinia Total Hip Implant II in the first half of 2009 and beginning clinical trials of this product candidate in the second half of 2009.
Our Knee Implant Product
Infinia Total Knee Implant
Our Infinia Total Knee Implant will incorporate our MC2 silicon nitride bearing components for the femoral condyle and will target the market for total knee implants estimated at $3.3 billion in the United States in 2006. The tibial tray will be made from traditional metal. The tibial insert will be made from polyethylene in a rotating platform design intended to give the knee implant a range of motion and flexion similar to the natural knee. As currently planned, this knee design will also feature an asymmetric design to closely simulate natural anatomy, together with a low profile metal tibial tray, to allow surgeons to implant the Infinia Total Knee Implant using minimally invasive surgical techniques. We anticipate that this knee implant will provide natural anatomic motion and will offer a low-wear knee replacement option, providing significantly improved longevity compared with current metal-on-polyethylene knee implants.
We are currently designing the Infinia Total Knee Implant. We anticipate submitting an IDE application for this implant system in the second half of 2009 and beginning clinical trials of this product candidate in the first half of 2010.
61
Our Ceramic Technologies
Since our inception, we have focused on the development of two technologies based on which we intend to commercialize advanced, high-performance orthopedic implant candidates:
| • |
We have developed a uniformly dense, micro-particle formulation of silicon nitride ceramic. We refer to the product candidates that we are developing based on this ceramic as our MC2, or Micro-Composite Ceramic, implants. |
| • |
We have developed implants that mimic the structure of natural bone by incorporating both a dense, load-bearing component and a porous component to promote bone attachment. We call these our CSC, or Cortico-cancellous Structured Ceramic, implants. |
Our MC2 Implants
The ceramic that we produce for our MC2 implants is made from silicon nitride formulated with dopants such as yttria and alumina. We believe we are the first company to engage in the development of ceramic-based spine and joint implants made from silicon nitride. We believe our implant candidates will provide a combination of high strength, fracture resistance, wear resistance and radiolucency that can overcome the limitations of currently available orthopedic implants.
Strength and Resistance to Fracture. We have conducted mechanical tests, following FDA guidelines, to compare the strength of 28 mm femoral heads made using silicon nitride ceramic material produced by a contract manufacturer to our specifications, with 28 mm femoral heads made using the strongest commercially available ceramic, Biolox Delta™. In these tests, we compared the “burst strength” of three designs of the silicon nitride femoral heads, made to the design specifications of three different orthopedic manufacturers, with the burst strength of comparably designed femoral heads made using Biolox Delta. We applied a load to the femoral heads, each of which was mounted on a typical hip implant stem, until the heads burst, which enabled us to measure the strength of the femoral heads and provided an indication of the fracture resistance. While 28 mm femoral heads are used by surgeons, as larger diameter femoral heads have become available, many surgeons prefer using these larger femoral heads to reduce the incidence of post-operative dislocation, one of the most common complications of hip replacement surgery. Because of this trend in favor of using larger diameter femoral heads, we also conducted burst strength tests of 38 mm femoral heads made from silicon nitride. The burst strength comparing the different femoral head designs are shown in the chart on the following page.
62
These tests demonstrated that the silicon nitride femoral heads had substantially greater burst strength than the femoral heads made with Biolox Delta. The silicon nitride femoral heads had burst strengths ranging from 71 kilo-Newtons, or kN, to 75 kN compared to average burst strengths ranging from 49 kN to 65 kN for the Biolox Delta femoral heads. We also have proven that larger silicon nitride femoral heads of 38 mm diameter have even greater burst strengths which averaged approximately 139 kN. The minimum FDA average burst strength requirement to legally market ceramic femoral heads is 46 kN.
We anticipate that the superior strength of the silicon nitride ceramic material that we intend to use for our implant products will provide us with a greater ability to develop implants that offer surgeons a wider size and design range than is possible with currently marketed ceramics. This, we believe, will provide surgeons with more flexibility than currently possible when choosing implants for patients, resulting in better clinical outcomes.
Wear Resistance. In 2000, we had a wear study performed by an independent laboratory on a similar silicon nitride ceramic material produced by a contract manufacturer to our specifications. In this study, we arranged for the laboratory to test silicon nitride femoral heads articulating against silicon nitride ceramic acetabular liners and cobalt-chromium metal alloy femoral heads articulating against silicon nitride ceramic acetabular liners. Using well-established protocols in a hip simulator, the silicon nitride ceramic bearings demonstrated:
| • | over 100 times lower wear than reported for metal-on-polyethylene hip bearings; |
| • | 20 times lower wear than reported for metal-on-cross-linked polyethylene hip bearings; |
| • | 10 times lower wear than reported for metal-on-metal hip bearings; and |
| • | comparable wear to that reported for existing ceramic-on-ceramic hip bearings. |
In 2006, we also had a separate wear study performed by the same independent laboratory to test silicon nitride ceramic femoral heads made from material produced by the same contract manufacturer, articulating against commercially available polyethylene liners. One of the conclusions from this simulator wear study was that the silicon nitride ceramic head-polyethylene liner combination exhibited low wear comparable to the low wear reported for the widely used combination of an alumina ceramic head articulating against a polyethylene liner.
63
As indicated in the clinical literature presented at the American Academy of Orthopedic Surgeons, ceramic-on-polyethylene bearings exhibit about 50% lower wear than metal femoral head-polyethylene liner combinations. The study also demonstrated the superior scratch resistance of the silicon nitride ceramic femoral heads compared with metal femoral heads, the latter having an 8-fold increase in surface roughness compared to the silicon nitride ceramic heads.
Radiolucency. We conducted a study to compare the imaging characteristics of discs made of metals such as titanium, plastics such as PEEK and silicon nitride using a cadaver human vertebral body. Images of the vertebral body and the discs were obtained using X-ray fluoroscopy, an imaging technique using a fluorescent screen to examine the internal structure of the body, computer tomography, or CT, an imaging technique for visualizing a three-dimensional image of the internal structure of the body, and MRI under identical conditions. We assessed the radiolucent characteristics of the discs in X-ray fluoroscopy images quantitatively, assessed the presence of scatter in CT scans qualitatively, and assessed distortion in MRI quantitatively. We found that in X-ray fluoroscopy, the metal discs did not permit visualization of the underlying bone of the vertebral body while PEEK was transparent, rendering its location difficult to determine. The silicon nitride disc had an intermediate radiolucency that rendered it visible as well as allowing a visual assessment of the underlying bone of the vertebral body. CT and MRI scans of the metal discs indicated the presence of distortion while silicon nitride and PEEK exhibited no scattering. The study thus demonstrated that the combination of partial radiolucency in X-ray fluoroscopy, and no distortion in CT and MRI scans would facilitate both placement of spinal spacers during surgery and post-operative monitoring of bone attachment.
MC2 Biocompatibility. We have conducted a full complement of required biocompatibility tests, following guidelines of the FDA and the International Standards Organization, or ISO. These tests confirmed that the silicon nitride ceramic produced by a contract manufacturer to our specifications met required biocompatibility standards for human use. We have submitted a master file to the FDA for this silicon nitride ceramic containing these test results. We intend to repeat the full battery of biocompatibility tests on silicon nitride produced in our manufacturing facility.
Our CSC Implants
Like natural bone, which has a cortical, or dense, load-bearing outer surface, and a cancellous, or porous, inner region, our CSC implant candidates have a solid load-bearing portion adjacent to a porous portion. By integrating both load-bearing and porous structures, our CSC implant candidates are designed to provide structural integrity while at the same time facilitating the attachment of surrounding bone to the implant. Our CSC implant candidates also incorporate a coating to promote bone attachment. While our CSC technology can be applied to any ceramic material, we are using it with our MC2 silicon nitride to develop implant candidates for applications where the promotion of secure bone attachment is important for successful implant fixation. We believe that the inertness of our silicon nitride ceramic material, coupled with the porous structure that mimics natural cancellous bone, will promote bone attachment. We also believe that this combination, together with a surface coating, may alleviate the need for bone morphogenic proteins to promote bone attachment. We have been issued two U.S. patents directed to our CSC technology. We also have exclusively licensed three U.S. patents and foreign counterparts, together with related know-how, directed to manufacturing processes for the production of porous ceramics for use in our orthopedic implants. These processes are versatile and allow us to manufacture our implants from our MC2 silicon nitride with a range of porosity and pore size that mimic natural cancellous bone.
Osteointegrative Properties. We arranged for a pilot animal study to be conducted using skeletally mature sheep to evaluate the ability of the porous portion of our CSC-designed implants to promote osteointegration, or the growth of new bone, within the test animals in a knee-defect model. In this study, we implanted porous cylinders made of a commonly used ceramic in the condyle, or lower end, of the femur, or thigh bone, of the sheep. This animal study showed the rapid osteointegration potential of the porous implants. The porous implants also had extensive new bone formation at and into the implant surface, and showed the presence of new bone at the center of the implants. We believe this study indicates the potential of our CSC implants to promote bone attachment.
64
Except for the studies and tests performed by third parties, our employees conducted the tests described above under the supervision of Ashok C. Khandkar, Ph.D., our Chief Executive Officer. Dr. Khandkar served as the principal contact for all tests conducted by third party investigators at our request. The wear study performed by an independent laboratory in 2000 was funded using a grant from the NIH and, pursuant to the requirements of the grant, Dr. Khandkar was designated as the principal investigator. As principal investigator, Dr. Khandkar assisted with the formulation of the overall hypotheses for the study and he and Aaron A. Hofmann, M.D., a member of our board of directors, consulted as to the methodology of this study. We made arrangements for the provision of femoral heads to the independent laboratory so that the laboratory could conduct the tests, and collect and report the data resulting from this study. We used our working capital to pay the costs associated with the pilot animal study and the 2006 wear study. Dr. Khandkar, in consultation with our senior employees within our research and development department, selected the independent laboratories that conducted the pilot animal study and the 2006 wear study. Employees within our research and development department, supervised by Dr. Khandkar, acted as the principal contacts for these studies. As was the case with respect to 2000 wear study, we arranged for the provision of femoral heads and animal implants to the laboratories for testing and the laboratories conducted the tests, and collected and reported the data resulting from these studies.
Sales and Marketing
Our marketing strategy will highlight our ceramic technologies and the design advantages of our product candidates. We intend to make strong distribution channels and technical education our strategic focal points.
Upon FDA clearance, we intend to market and sell our lead spinal products in the United States using a hybrid distribution network that includes a combination of experienced, independent sales agents with strong, existing surgeon relationships and a direct sales force in selected markets. A similar hybrid sales force will also be used to market our hip and knee reconstructive products. We have begun to sign agreements with independent sales agents. We intend to employ a clinically experienced technical support team consisting of health care professionals to assist in the training of clinicians and their staff.
We intend to use our surgeon advisors to help implement an awareness campaign for educating other spine and reconstructive joint surgeons about our products. As part of this campaign we plan to provide educational materials to treating physicians, referring physicians and patients. We also intend to organize regional training seminars where our product and training managers, engineers, and sales and marketing staff, together with our surgeon advisors, will educate other surgeons and sales agent support staff in the use of our products.
In selected international markets, such as Europe, Japan, Australia and Canada, we may also seek to establish collaborations with leading orthopedic companies where we believe that a large, well-established partner may provide better access to those markets. In addition, we may establish collaborations in the United States under circumstances where access to a larger sales and marketing organization may help to expand the market or accelerate penetration for selected products.
Product Manufacturing
In order to control the quality, cost and availability of our silicon nitride implants, we are developing our own manufacturing capabilities. Until August 2006, we conducted our manufacturing operations in a 3,378 square-foot pilot and prototype manufacturing facility at our present location in Salt Lake City, Utah, and we used third parties to produce some components of our other ceramic product candidates, such as silicon nitride ball blanks for femoral heads. During the past two years, we have also been developing scaled-up manufacturing processes and building out of our manufacturing facility, certified under the ISO 13485 standard for medical devices, located in an approximately 17,000 square-foot facility near our corporate offices. We expect our manufacturing facility to be fully operational by the end of 2007. It will be equipped with state-of-the-art, computerized mixing equipment, sintering furnaces, robotic machining centers and other testing equipment that will enable us to control the entire process for manufacturing our ceramic implants from powder form to devices ready for sterilization and packaging. To our knowledge, we will be the only vertically integrated orthopedic implant company in the world with the capability to make spine and joint implants from silicon nitride.
65
Our ceramic manufacturing strategy includes the purchase of raw materials from one or more vendors which are ISO registered and approved by us. These raw materials, consisting of silicon nitride ceramic powder and dopant chemical compounds, are characterized and tested in our facility in accordance with our specifications, and then blended to formulate our silicon nitride material. Subsequently, we form the silicon nitride material into implant components using specialized processing equipment, including computer-controlled machining centers and sintering furnaces. In addition, for our CSC implants, we have licensed, on a worldwide exclusive basis, a patented technology and related know-how to manufacture porous ceramics.
We plan to rely exclusively on third-parties for the manufacture of products or components made from metals or plastics, including our Valeo Cervical Plate System, Valeo Pedicle Screw System, the metal components of our Infinia hip and knee implants, and surgical instrumentation sets. Our outsourcing strategy is targeted at using contract manufacturers that are FDA registered and which meet the ISO 13485 certification standard. We believe the use of third-party sources for metals or plastics will reduce our capital investment requirements and allow us to strategically focus our resources on the development of our product candidates.
We are currently working with our ceramic raw material vendors and parts suppliers to ensure that they can meet our commercialization requirements. We are currently developing and qualifying alternative sources of supply for our raw materials.
Intellectual Property
We rely on a combination of patents, trademarks, trade secrets and other intellectual property laws, nondisclosure agreements, proprietary information ownership agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must continue to develop and maintain the proprietary aspects of our technologies.
Currently, we have four issued U.S. patents, 12 pending U.S. patent applications, and ten pending foreign patent applications. Our U.S. Patent No. 6,881,229, issued April 19, 2005, is directed to an articulating joint prosthesis having a cobalt chromium head and a cup made from a high strength, high toughness doped silicon nitride ceramic. Our U.S. Patent Nos. 6,846,327, issued January 25, 2005, and 6,790,233, issued September 14, 2004, are directed to a bone graft and spinal fixation cage having a cortico-cancellous structure with a bioactive and resorbable surface coating. Our U.S. Patent No. 6,994,727, issued February 7, 2006, is directed to a novel prosthesis for use in replacing a spinal disc. Our issued patents begin to expire in 2022, with the last of these patents expiring in 2023.
Our pending patent applications are directed to additional aspects of our technologies including, among other things:
| • |
additional embodiments of implants using our MC2 silicon nitride in one or more implant components; |
| • |
additional embodiments of cortico-cancellous structured implants using our CSC technology, including such implants without a bioactive, resorbable coating; |
| • | designs for cervical plates; |
| • | designs for pedicle screws; |
| • | designs for cervical disc implants; |
| • | designs for intervertebral spacers; |
| • | designs for hip implants; and |
| • | designs for knee implants. |
We also have exclusively licensed from Dytech Corporation Ltd. three U.S. patents and foreign counterparts, together with related know-how, directed to manufacturing processes for the production of porous ceramics for use in our orthopedic implants. Individual patents extend for varying periods depending on the
66
effective date of filing of the patent application or the date of patent issuance, and the legal term of the patents in the countries in which they are obtained. Generally, since our U.S. patent applications were filed on or after June 8, 1995, our patents issued, and those to be issued, in the United States are effective for 20 years from the earliest effective filing date. The term of our foreign patents will vary in accordance with provisions of applicable local law, but typically will be 20 years from the earliest effective filing date.
We also expect to rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our intellectual property position. However, trade secrets are difficult to protect. We seek to protect the trade secrets in our proprietary technology and processes, in part, by entering into confidentiality agreements with commercial partners, collaborators, employees, consultants, scientific advisors and other contractors and into invention assignment agreements with our employees and some of our commercial partners and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of the technologies that are developed.
Industry Competition
The orthopedic implant industry is highly competitive. We believe our main global competitors in the spine implant market include Medtronic Spinal and Biologics, a subsidiary of Medtronic, Inc.; Synthes, Inc.; DePuy Spine, Inc., a subsidiary of Johnson & Johnson; Stryker Spine, a division of Stryker Corporation; Biomet Spine and Biomet Trauma, a subsidiary of Biomet, Inc.; and Zimmer Spine, a subsidiary of Zimmer Holdings, Inc., which, in 2006, together accounted for over 80% of the market. We believe our main competitors in the hip and knee implant market are Zimmer Holdings, Inc.; DePuy Orthopaedics, Inc., a subsidiary of Johnson & Johnson; Stryker Orthopaedics, a division of Stryker Corporation; Biomet, Inc.; and Smith & Nephew Orthopaedics, a subsidiary of Smith & Nephew plc, which, in 2006, together accounted for over 80% of the market.
Competition within the industry is primarily based on technology, innovation, product quality, and the product awareness and acceptance by orthopedic surgeons. Our principal competitors have substantially greater financial, technical and marketing resources, as well as significantly greater manufacturing capabilities, than we do, and they may succeed in developing products that render our products non-competitive. Our ability to compete successfully will depend upon our ability to develop innovative products with advanced performance features based on our ceramic technologies.
We anticipate that orthopedic companies will also seek to introduce new ceramic-based implants to compete with ours. Presently, these companies buy ceramic components from manufacturers such as Ceramtec, Metoxit, Morgan-Matroc, Kyocera and NTK. These companies manufacture and provide ceramic femoral heads on an original equipment manufacturer, or OEM, basis to orthopedic implant companies such as Stryker, DePuy and Zimmer. We will seek to compete with their products based on the performance advantages offered by our silicon nitride-based ceramic technologies.
Government Regulation of Medical Devices
Governmental authorities in the United States, at the federal, state and local levels, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of products such as those we are developing. Failure to obtain approval to market our products under development and to meet the ongoing requirements of these regulatory authorities could prevent us from marketing and continuing to market our products.
United States
In the United States, before a new medical device can be marketed, its manufacturer must either obtain marketing clearance through a premarket notification under Section 510(k) of the Federal Food, Drug and Cosmetic Act or marketing approval of a premarket approval application, or PMA. User fees, which increase each year and which are specific for the type of submission that is made, must be paid to the FDA at the time that the 510(k) or PMA is submitted. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the
67
FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls, including labeling, premarket notification and adherence to the quality systems regulation, or QSR, which sets forth device-specific good manufacturing practices. Class II devices are subject to general controls and special controls, including performance standards and post-market surveillance. Class III devices are subject to most of the previously identified requirements as well as to premarket approval.
A 510(k) premarket notification must demonstrate that the device in question is substantially equivalent to another legally marketed device, or predicate device, that does not require premarket approval. In evaluating the 510(k), the FDA must determine that (i) the device has the same intended use as the predicate device and (ii) has the same technological characteristics as the predicate device, or (a) has different technological characteristics, (b) the data submitted establishes that the device is substantially equivalent and contains information, including clinical data if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device and (c) the device does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but a minority do require clinical data support. The FDA is supposed to issue a decision letter within 90 days if it has no additional questions or send a first action letter requesting additional information within 75 days; however, the FDA does not always meet the applicable performance goal review time. In addition, requests for additional data, including clinical data, will increase the time necessary to review the notice. Most Class I devices and many Class II devices are exempt from the 510(k) requirement. If the FDA does not agree that the new device is substantially equivalent to the predicate device, the new device will be classified in Class III, and the manufacturer must submit a PMA or may, depending on the nature of the device, petition the FDA to make a risk-based determination of the new device and reclassify the new device in Class I or II. Modifications to a 510(k)-cleared medical device may require the submission of another 510(k) or a PMA if the changes could significantly affect the safety or effectiveness or constitute a major change in the intended use of the device.
Modifications to a 510(k)-cleared device frequently require the submission of a traditional 510(k), but modifications meeting certain conditions may be candidates for FDA review under a Special 510(k). If a device modification requires the submission of a 510(k), but the modification does not affect the intended use of the device or alter the fundamental scientific technology of the device, then summary information that results from the design control process associated with the cleared device can serve as the basis for clearing the application. A Special 510(k) allows a manufacturer to declare conformance to design controls without providing new data. When the modification involves a change in material, the nature of the “new” material will determine whether a traditional or Special 510(k) is necessary. For example, in its Device Advice on How to Prepare a Special 510(k), the FDA uses the example of a change in a material in a finger joint prosthesis from a known metal alloy to a ceramic that has not been used in a legally marketed predicate device as a type of change that should not be submitted as a Special 510(k). However, if the “new” material is a type that has been used in other legally marketed devices within the same classification for the same intended use, a Special 510(k) is appropriate. The FDA gives as an example a manufacturer of a hip implant who changes from one alloy to another that has been used in another legally marketed predicate. Special 510(k)s are typically processed within thirty (30) days of receipt.
The PMA process is more complex, costly and time consuming than the 510(k) clearance procedure. A PMA must be supported by more detailed scientific evidence than a 510(k) notice, including clinical data to demonstrate the safety and efficacy of the device. If the device is determined to present a “significant risk,” the manufacturer may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval from the FDA. Such clinical trials are also subject to the review, approval and oversight of an institutional review board, or IRB, at each institution at which the clinical trial will be performed. The clinical trials must be conducted in accordance with applicable regulations, including but not limited to the FDA’s IDE regulations. Upon completion of the clinical trials, and assuming that the results indicate that the product is safe and effective for its intended use, the manufacturer will then submit a PMA. The FDA has 45 days after a PMA is submitted to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for
68
PMAs and may issue a decision letter as a first action on a PMA within 180 days of filing, but if it has questions, it will likely issue a first major deficiency letter within 150 days. It may also refer the PMA to an FDA advisory committee for additional review, and will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, either of which could extend the 180 day target for a response. The FDA may also inspect the investigational sites to ensure compliance with the IDE and other applicable regulations governing the conduct of the trial. While the FDA’s ability to meet its performance goals has generally improved during the past few years, it may not meet these goals in the future. A PMA can take several years to complete and there is no assurance that any submitted PMA will ever be approved. Even when approved, the FDA may limit the indication for which the medical device may be marketed or to whom it may be sold. In addition, the FDA may request additional information or request the performance of additional clinical studies as a condition of approval or after the PMA is approved. Changes to the device or its manufacturing process may require the prior approval of a supplemental PMA.
Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
| • | compliance with the QSR, which require manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process; |
| • | labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | warning letters; |
| • | fines, injunctions, and civil penalties; |
| • | recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusal to grant 510(k) clearance or PMA approvals of new products; |
| • | withdrawal of 510(k) clearance or PMA approvals; and |
| • | criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled or unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our subcontractors.
International Regulation
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. For example, the primary regulatory authority with respect to medical devices in Europe is that of the European Union, which consists of about twenty-four countries encompassing most of the major countries in Europe. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially
69
distributed throughout the European Union, although actual implementation of these directives may vary on a country-by-country basis. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body.” This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s product. An assessment by a Notified Body in one country within the European Union is required in order for a manufacturer to distribute the product commercially throughout the European Union.
Compliance with Fraud and Abuse Laws
Once our product candidates are commercialized, we must comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws, rules and regulations. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare, Medicaid, Veterans Administration health programs, workers’ compensation programs and TRICARE. We operate our business to be in material compliance with such laws, rules and regulations.
We have entered into agreements with certain surgeons for assistance with the design of our products, some of whom we anticipate may make referrals to us or order our products. A majority of these agreements contain provisions for the payments of royalties and/or stock options. In addition, some surgeons currently own shares of our stock and other surgeons may be offered shares as part of this offering under our directed share program as described in the “Underwriters” section of this prospectus. These transactions were, and will be, structured with the intention of complying with all applicable laws, including fraud and abuse laws. Despite this intention, the laws in this area are both broad and vague, and it is often difficult or impossible to determine how the laws will be applied. Accordingly, there can be no assurance that a particular government agency or court would determine our practices to be in full compliance with such laws. We could be materially impacted if regulatory or enforcement agencies or courts interpret our financial arrangements with surgeons to be in violation of these fraud and abuse laws.
Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits persons from knowingly or willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce:
| • | the referral of an individual for a service or product for which payment may be made by Medicare, Medicaid or any other government-sponsored healthcare program; or |
| • | purchasing, ordering, arranging for, or recommending the ordering of, any service or product for which payment may be made by a government-sponsored healthcare program. |
The definition of “remuneration” has been broadly interpreted to include anything of value, including such items as gifts, certain discounts, waiver of payments, and providing anything at less than its fair market value. In addition, several courts have interpreted the law to mean that if “one purpose” of an arrangement is intended to induce referrals, the statute is violated.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, the Office of Inspector General of the Department of Health and Human Services, or OIG, has issued regulations, commonly known as “safe harbors.” These safe harbors set forth certain requirements that, if fully met, will assure healthcare providers, including medical device manufacturers, that they will not be prosecuted under the Anti-Kickback Statute. Although full compliance with these safe harbor provisions ensures against prosecution under the Anti-Kickback Statute, full compliance is often difficult and the failure of a transaction or arrangement to fit within a specific safe harbor
70
does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the Anti- Kickback Statute will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. The statutory penalties for violating the Anti-Kickback Statute include imprisonment for up to five years and criminal fines of up to $25,000 per violation. In addition, through application of other laws, conduct that violates the Anti-Kickback Statute can also give rise to False Claims Act lawsuits, civil monetary penalties and possible exclusion from Medicare and Medicaid and other federal healthcare programs. In addition to the Federal Anti-Kickback Statute, many states have their own anti-kickback laws. Often, these laws closely follow the language of the federal law, although they do not always have the same scope, exceptions, safe harbors or sanctions. In some states, these anti-kickback laws apply not only to payment made by a government health care program but also with respect to other payors, including commercial insurance companies.
One form of a financial arrangement that is subject to the Anti-Kickback Statute and other fraud and abuse laws is the so-called gainsharing program. While there is no fixed definition of gainsharing, the term typically refers to an arrangement in which a hospital gives physicians a share of any reduction in the hospital’s costs attributable in part to the physician’s efforts. Such cost reduction activities may relate to certain surgical procedures and surgeons may be asked to select less expensive devices to use in their surgeries, with the surgeons then sharing in the cost savings to the hospital.
Government officials have focused recent kickback enforcement efforts on, among other things, the sales and marketing activities of healthcare companies, including medical device manufacturers, and recently have brought cases against individuals or entities with personnel who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business. This trend is expected to continue. Settlements of these cases by healthcare companies have involved significant fines and/or penalties and in some instances criminal plea agreements. We are also aware of governmental investigations of some of the largest orthopedic device companies reportedly focusing on consulting and service agreements between these companies and orthopedic surgeons. These developments are ongoing and we cannot predict the effects they will have on our business.
Physician Self-Referral Laws
The federal ban on physician self-referrals, commonly known as the “Stark Law,” prohibits, subject to certain exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician has any financial relationship with the entity. The Stark Law also prohibits the entity receiving the referral from billing for any good or service furnished pursuant to an unlawful referral, and any person collecting any amounts in connection with an unlawful referral is obligated to refund these amounts. A person who engages in a scheme to circumvent the Stark Law’s referral prohibition may be fined up to $100,000 for each such arrangement or scheme. The penalties for violating the Stark Law also include civil monetary penalties of up to $15,000 per service and possible exclusion from federal healthcare programs. In addition to the Stark Law, many states have their own self-referral laws. Often, these laws closely follow the language of the federal law, although they do not always have the same scope, exceptions, safe harbors or sanctions. In some states these self-referral laws apply not only to payment made by a federal health care program but also with respect to other payors, including commercial insurance companies. In addition, some state laws require physicians to disclose any financial interest they may have with a healthcare provider to their patients when referring patients to that provider even if the referral itself is not prohibited.
Other Fraud and Abuse Laws
The federal False Claims Act, or FCA, prohibits any person from knowingly presenting, or causing to be presented, a false claim or knowingly making, or causing to be made, a false statement to obtain payment from the federal government. Those found in violation of the FCA can be subject to fines and penalties of three times
71
the damages sustained by the government, plus mandatory civil penalties of between $5,500 and $11,000 for each separate false claim. Actions filed under the FCA can be brought by any individual on behalf of the government, a qui tam action, and this individual, known as a “relator” or, more commonly, as a “whistleblower,” may share in any amounts paid by the entity to the government in damages and penalties or by way of settlement. In addition, certain states have enacted laws modeled after the FCA, and this legislative activity is expected to increase. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies, including medical device manufacturers, to defend false claim actions, pay damages and penalties or be excluded from Medicare, Medicaid or other federal or state healthcare programs as a result of investigations arising out of such actions.
The OIG also has authority to bring administrative actions against entities for alleged violations of a number of prohibitions, including the Anti-Kickback Statute and the Stark Law. The OIG may seek to impose civil monetary penalties or exclusion from the Medicare, Medicaid and other federal healthcare programs. Civil monetary penalties can range from $2,000 to $50,000 for each violation or failure plus, in certain circumstances, three times the amounts claimed in reimbursement or illegal remuneration. Typically, exclusions last for five years.
In addition, we must comply with a variety of other laws, such as laws prohibiting false claims for reimbursement under Medicare and Medicaid and laws prohibiting gainsharing programs, all of which can also be triggered by violations of federal anti-kickback laws; the Health Insurance Portability and Accounting Act of 1996, which makes it a federal crime to commit healthcare fraud and make false statements; and the Federal Trade Commission Act and similar laws regulating advertisement and consumer protections.
Third-Party Reimbursement
Because we expect to receive payment directly from hospitals and surgical centers, we do not anticipate relying directly on payment for any of our products from third-party payors, such as Medicare, Medicaid, private insurers and managed care companies. However, our business will be affected by policies administered by federal and state governmental authorities, such as Medicare and Medicaid, as well as private payors, which often follow the policies of these public programs. For example, our business will be indirectly impacted by the ability of a hospital or medical facility to obtain coverage and third-party reimbursement for procedures performed using our products. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not medically necessary, was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. For example, on May 16, 2006, the Centers for Medicare and Medicaid Services issued a national coverage decision denying Medicare coverage for DePuy’s CHARITE™ prosthetic intervertebral disc implant for patients over 60 years old. This national coverage decision is under reconsideration and a decision is expected later this year. A national coverage decision denying Medicare coverage could result in private insurers and other third party payors denying coverage for this and similar products.
For inpatient and outpatient spine fracture reduction procedures, including those that will involve use of our products once approved, Medicare reimburses hospitals at a prospectively determined amount, called diagnosis related groups, or DRGs, for inpatient treatment and ambulatory payment classifications for outpatient treatment. Each of these DRG codes is associated with a level of payment and is adjusted from time to time, usually annually. DRG payments are intended to cover most of the non-physician hospital costs incurred in connection with the applicable diagnosis and related procedures. Implant products, such as those we plan to sell, represent part of the total procedure costs, while labor, hospital room and board and other supplies and services represent the balance of those costs. However, the DRG payment amounts are typically set independently of a particular hospital’s actual cost for treating a patient and implanting a device. Thus, the payments that a hospital would receive for a particular procedure would not typically be based on the cost of our products.
72
Medicare has established a number of DRGs for inpatient procedures that involve the use of products similar to ours. Although Medicare has authority to create special DRGs for hospital services that more properly reflect the actual costs of expensive or new-technology devices implanted as part of a procedure, it has recently declined to do so for DePuy’s CHARITE™ prosthetic intervertebral disc implant.
We believe that orthopedic implants generally have been well received by third-party payors because of the ability of these implants to greatly reduce long-term health care costs for patients with degenerative joint disease. However, coverage and reimbursement policies vary from payor to payor and are subject to change. As discussed above, hospitals that purchase medical devices for treatment of their patients generally rely on third-party payors to reimburse all or part of the costs and fees associated with the procedures performed with these devices. Both government and private third-party coverage and reimbursement levels are critical to new product acceptance. Neither hospitals nor spine surgeons are likely to use our products if they do not receive reimbursement adequate to cover the cost of these procedures.
While it is expected that hospitals will be able to obtain coverage for procedures using our products, the level of payment available to them for such procedures may change over time. Governmental payors such as Medicare and Medicaid closely regulate provider payment levels and have sought to contain, and sometimes reduce, payment levels. Commercial payors and managed care plans frequently follow government payment policies, and are likewise interested in controlling increases in the cost of medical care. These third-party payors may deny payment if they determine that a procedure was not medically necessary, a device used in a procedure was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication.
In addition, some payors are adopting pay-for-performance programs that differentiate payments to health care providers based on the achievement of documented quality-of-care metrics, cost efficiencies, or patient outcomes. These programs are intended to provide incentives to providers to find ways to deliver the same or better results while consuming fewer resources. As a result of these programs, and related payor efforts to reduce payment levels, hospitals and other providers are seeking ways to reduce their costs, including the amounts they pay to medical device suppliers. Adverse changes in payment rates by payors to hospitals could adversely impact our ability to market and sell our products and negatively affect our financial performance.
In international markets, healthcare payment systems vary significantly by country and many countries have instituted price ceilings on specific product lines. There can be no assurance that our products will be considered cost-effective by third-party payors, that reimbursement will be available or, if available, that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably.
Member countries of the EU offer various combinations of centrally financed health care systems and private health insurance systems. The relative importance of government and private systems varies from country to country. The choice of devices is subject to constraints imposed by the availability of funds within the purchasing institution. Medical devices are most commonly sold to hospitals or health care facilities at a price set by negotiation between the buyer and the seller. A contract to purchase products may result from an individual initiative or as a result of a competitive bidding process. In either case, the purchaser pays the supplier, and payment terms vary widely throughout the EU. Failure to obtain favorable negotiated prices with hospitals or health care facilities could adversely affect sales of our products.
Employees
As of June 30, 2007, we had 37 full-time employees, including four who hold Ph.D. degrees. Of our 37 employees, five are employed in administration, 29 in manufacturing and research and development, and three in sales and marketing. We believe that our success will depend, in part, on our ability to attract and retain qualified personnel. We have never experienced a work stoppage due to labor difficulties and believe that our relations with our employees are good. None of our employees is represented by a labor union.
73
Legal Matters
We are currently not a party to any material legal proceedings.
Facilities
Our corporate office and our manufacturing facilities are located in Salt Lake City, Utah. We believe that our existing facilities are adequate for our current needs. The table below provides selected information regarding our facilities, all of which are leased.
| Location |
Use |
Approximate Square Footage |
Lease Expiration | |||
| Salt Lake City, Utah |
Corporate headquarters, research and development and administrative offices | 9,505 | August 2009 | |||
| Salt Lake City, Utah |
Manufacturing | 17,439 | April 2011 | |||
Plan of Operations
We believe that the net proceeds from our initial public offering, our anticipated future revenue and our cash, cash equivalent marketable securities balances and interest we earn on these balances, will be sufficient to meet our anticipated cash requirements through the end of 2009. During the period between the date of this prospectus and June 30, 2008, ignoring the impact of potential revenue resulting from sales of three of our lead spinal implant products before June 30, 2008, we believe that it will not be necessary to raise additional funds to meet the expenditures required to operate our business, including expenditures for:
| • | building sales, marketing and distribution capabilities for our spinal implant products in anticipation of commercialization of some of our lead spinal products, which we plan to launch beginning in the first half of 2008; |
| • | the scale-up of our manufacturing operations to commercial level; |
| • | the continuation of our research and development activities on our pipeline of products in development; |
| • | expenditures relating to our compliance with applicable laws and regulations associated with being a publicly traded company; and |
| • | expenditures relating to our seeking regulatory clearance and approval of our lead products, including the commencement of clinical testing of some of our lead products. |
74
Directors and Executive Officers
Our directors and executive officers and their respective ages and positions as of June 30, 2007 are as follows:
| Name |
Age | Position | ||
| Max Link, Ph.D.(1)(2) |
66 | Chairman of the Board of Directors | ||
| Ashok C. Khandkar, Ph.D. |
50 | Director and Chief Executive Officer | ||
| Aaron A. Hofmann, M.D. |
57 | Director | ||
| Lawrence D. Dorr, M.D.(3) |
66 | Director | ||
| Gregg R. Honigblum |
44 | Director | ||
| Rohit Patel(1)(2)(3) |
66 | Director | ||
| Bradford S. Goodwin(1)(3) |
52 | Director | ||
| Warionex (“Jose”) Belen |
57 | President | ||
| Bryan J. McEntire |
55 | Vice President of Manufacturing and Research | ||
| Reyn E. Gallacher |
44 | Vice President of Finance, Chief Financial Officer and Assistant Secretary | ||
| Kenneth W. Ludwig, Jr. |
55 | Vice President of Marketing | ||
| Robert M. Wolfarth |
44 | Director of Regulatory Affairs and Quality Assurance | ||
| (1) | Member of our audit committee |
| (2) | Member of our nominating and corporate governance committee |
| (3) | Member of our compensation committee |
The following is a brief summary of the background of each of our directors and executive officers.
Max Link, Ph.D. has served as the Chairman of our board of directors since October 2003. Dr. Link served as Chairman of the Board and Chief Executive Officer of Centerpulse AG, the largest orthopedics company in Europe, from March 2002 until October 2003, when Centerpulse was acquired by Zimmer Holdings, Inc. Prior to joining Centerpulse, Dr. Link served as the Chief Executive Officer at Corange Limited/Boehringer Mannheim and as Chairman and Chief Executive Officer at Sandoz Pharmaceuticals (now part of Novartis). Since 1994, Dr. Link has been actively involved as a director in development stage companies in the healthcare and biopharmaceutical field both in the United States and in Europe, including Human Genome Sciences, Inc., Alexion Pharmaceuticals, Inc., Celsion Corporation and Discovery Laboratories, Inc. Dr. Link received his Ph.D. in economics from the University of St. Gallen, Switzerland in 1970.
Ashok C. Khandkar, Ph.D. is our co-founder and has been a member of our board of directors since 1996. Dr. Khandkar has been our Chief Executive Officer since we hired him for that position in February 2000. He also served as our President from September 2003 through December 2006. Dr. Khandkar has more than 20 years of experience in senior managerial positions in ceramics development and manufacturing with responsibility for finance, strategic planning and business development. Dr. Khandkar is an inventor on 24 U.S. and international patents. Prior to becoming our Chief Executive Officer, Dr. Khandkar served as a Vice President with Ceramatec, where he started its oxide fuel cell program. He also served as Chief Technology Officer of SOFCo, a joint venture between McDermott Inc. and Ceramatec, where he managed a multi-disciplinary team of engineers, scientists, and manufacturing professionals. Dr. Khandkar has authored more than 30 papers related to ceramics technology. He has served as the vice-chair of the High Temperature Materials Division of the Electrochemical Society and is an Adjunct Associate Professor in the Materials Science and Engineering Department of the University of Utah in Salt Lake City, Utah. Dr. Khandkar earned his Ph.D. in materials science from Arizona State University in 1985.
75
Aaron A. Hofmann, M.D. is our co-founder and has been a member of our board of directors since 1996. Dr. Hofmann is a nationally and internationally recognized orthopedic surgeon, known for his accomplishments in developing total hip and knee replacement systems, innovative surgical approaches in hip and knee surgery and basic research on human bone dynamics. Dr. Hofmann holds 14 patents, many of which are directed at inventions involving hip and knee implants. Since 2003, Dr. Hofmann has been a design surgeon for Zimmer Holdings, Inc., the largest orthopedics company in the world. Dr. Hofmann, working with a team of orthopedic surgeons, helped design Zimmer’s new gender knee specifically designed for women. Since 1992, Dr. Hofmann has been a Professor of Orthopedic Surgery at the University of Utah School of Medicine and Chief of Orthopedics for the Veteran Affairs Medical Center in Salt Lake City, Utah since 1988. He earned his M.D. at the Southwestern Medical School in Dallas, Texas, was a resident at Parkland Memorial Hospital in Dallas, Texas, and a Joint Reconstruction Fellow at the Montreal General Hospital. Dr. Hofmann is a diplomate of the American Board of Orthopaedic Surgery and an active member of the American Orthopaedic Association, the American Academy of Orthopaedic Surgeons, the Orthopaedic Research Society, the Society for Arthritic Joint Surgery and the Knee Society.
Lawrence D. Dorr, M.D. has been a member of our board of directors since November 2006. Dr. Dorr is a nationally and internationally recognized orthopedic surgeon, known for this accomplishments in developing total hip and knee replacement systems, innovative minimally invasive surgical approaches and basic research on human bone dynamics. Dr. Dorr founded The Arthritis Institute in February 2001 and has been its Medical Director since then, performing more than 3,500 hip and knee replacements in the past decade. Prior to that he established the Arthritis Service at the Kerlan-Jobe Orthopaedic Clinic in 1983, and later founded the Center for Arthritis and Joint Implant Surgery at the University Hospital at the University of Southern California in 1992. Dr. Dorr served as the President of the American Association of Hip and Knee Surgeons, or AAHKS, from 1993 to 1994 and is the incoming President of the Hip Society in November 2007. Dr. Dorr received the Lifetime Humanitarium Award from the AAOS for Operation Walk in 2005. Dr. Dorr earned his medical degree and master’s degree from the University of Iowa. He completed his residency at the L.A. County-University of Southern California Medical Center, where he later served as a full-time teaching member of the Department of Orthopaedics at the University of Southern California.
Gregg R. Honigblum has been a member of our board of directors since December 2006. Mr. Honigblum has more than 20 years of experience as a financier for emerging growth companies primarily in the healthcare area. He has been instrumental in providing early stage financing for companies such as Myriad Genetics, Inc. and Acacia Biosciences, Inc., which merged with Rosetta Inpharmatics, Inc. and is now a wholly owned subsidiary of Merck. Since 2001, Mr. Honigblum has been the Chief Executive Officer and founder of Creation Capital LLC, an investment banking firm specializing in the financing and development of early stage medical device and biotechnology companies. Creation Capital has served as the placement agent for our private financings and has provided, through its client base, more than $44 million of equity capital. Mr. Honigblum holds a B.A. in economics from the University of Texas at Austin.
Rohit Patel has been a member of our board of directors since 2003 and an advisor to Amedica since its inception. Since May 2004, Mr. Patel served as the Chief Executive Officer of Ellis, Inc., a technology company developing and marketing English language learning software until retiring from Ellis in October 2006 after successfully transitioning the company to its new owner, Pearson Publishing. Prior to joining Ellis, from 2002 to 2004, he served as a consultant to NIIT, Ltd. of India and Ellis. From September 1996 to December 2001, Mr. Patel served as President of BNA Communications, Inc. and Executive Director of BNA Inc. and was responsible for three of its publishing divisions. BNA, Inc. is a leading publisher of information and analysis products for professionals in law, tax, business, and government based in Washington, D.C. and is the largest employee-owned company in the United States. From 1987 to 1995, Mr. Patel held various positions, including Chief Executive Officer, with Wicat Systems, Inc., a global developer of flight training and desk-top simulation software. Mr. Patel has an M.B.A. from Michigan State University and a M.S. in engineering from the University of Wisconsin.
76
Bradford S. Goodwin has been a member of our board of directors since May 2007. Mr. Goodwin is currently a director of PDL BioPharma and Rigel, Inc. He was the Chairman of the Board of CoTherix, Inc., a publicly traded company focused on pulmonary arterial hypertension, until its sale to Actelion Pharmaceuticals in early 2007. From 2001 to 2006, Mr. Goodwin was Chief Executive Officer and Director of Novacea, Inc., a publicly traded biopharmaceutical company focused on in-licensing, developing and commercializing novel therapies for cancer. From April 2000 to July 2001, Mr. Goodwin was President, Chief Operating Officer and founder of Collabra Pharma, a company focused on pharmaceutical product licensing and development. From April 1987 to February 2000, he held various senior executive positions with Genentech, Inc., including Vice President of Finance, and was responsible for treasury, purchasing, risk management, real estate, controllership, tax and long-range planning. For ten years prior to joining Genentech, Mr. Goodwin worked at Price Waterhouse LLP, now PricewaterhouseCoopers LLP, as a certified public accountant, serving ultimately as Senior Audit Manager. Mr. Goodwin also served on expert advisory committees of the American Institute of Certified Public Accountants, the Financial Accounting Standards Board and the International Accounting Standards Board. Mr. Goodwin holds a B.S. in business administration from the University of California, Berkeley.
Warionex (“Jose”) Belen has served as our President since December 2006. Mr. Belen held the position of Vice President of Products from May 2005 until December 2006. Mr. Belen has 34 years of experience in the orthopedics industry in engineering, marketing and national sales accounts. For 22 years prior to joining Amedica, Mr. Belen held various positions with Centerpulse AG prior to its acquisition by Zimmer Holdings, Inc. Prior to joining Amedica, until April 2005, he served as Director of Surgeon Consulting Services for Centerpulse, where he formalized product development agreements and managed surgeons who were members of Centerpulse AG’s product development teams.
Bryan J. McEntire has served as our Vice President of Manufacturing since August 2004 and as our Vice President of Research since December 2006. Mr. McEntire has more than 30 years of experience in advanced ceramic product development, quality engineering and manufacturing. Prior to joining Amedica, Mr. McEntire served as a senior director of supply chain management at Applied Materials in Silicon Valley from April 1998 to August 2004, where he managed the supply chain, which included the negotiation of supply contracts, and supervision of vendor production of various parts, including precision ceramic parts, which were integrated into the capital equipment made and sold by Applied Materials. Prior to joining Applied Materials, he was the General Manager of Norton Advanced Ceramics, a division of Saint-Gobain Industrial Ceramics Corporation, from 1993 to 1998, where he managed four ceramic product manufacturing plants in the United States.
Reyn E. Gallacher joined Amedica in January 2006 as our Controller, and has served as our Vice President of Finance since January 2007, and as our Chief Financial Officer and Assistant Secretary since March 2007. Mr. Gallacher has more than 20 years of financial and management experience working with both public and private companies, with responsibilities for financings as well as mergers and acquisitions. Prior to joining Amedica, Mr. Gallacher served as an internal auditor with Deseret Management Corporation from 2004 to 2006. Mr. Gallacher served as the Corporate Controller of AMI Semiconductor, a publicly traded semiconductor company, from 2003 to 2004. Mr. Gallacher also was the Corporate Controller of Pharmadign, Inc. from 2001 to 2003 and TrainSeek, Inc. from 2000 to 2001. From 1994 to 1999, Mr. Gallacher served in various financial and business management capacities with TheraTech, Inc., a transdermal drug delivery company, playing a key role with the due diligence and valuation work completed as part of the acquisition of TheraTech by Watson Pharmaceuticals. From 1987 to 1994, Mr. Gallacher served in various roles, most recently as a senior manager with KPMG in both their Salt Lake City, Utah and Montvale, New Jersey offices. While with KPMG, Mr. Gallacher specialized in working with small to mid-size companies in the health care and high technology industries. Mr. Gallacher holds a B.S. in accounting from the University of Utah and an M.B.A. from Weber State University.
Kenneth W. Ludwig, Jr. joined Amedica in May 2006 with 25 years of experience in the orthopedics devices industry, and 32 years of overall medical industry experience. In December 2006, Mr. Ludwig was promoted as our Vice President of Marketing. From 2001 to May 2006, he served as Vice President, Orthopedics at Aesculap, Inc. Between 1992 and 2001, Mr. Ludwig served as Vice President, Marketing and Vice President, Sales and Marketing - Spine at Encore Orthopedics, of which he was a co-founder. Mr. Ludwig began his career with
77
Howmedica in the early 1980’s and subsequently joined Intermedics Orthopedics in 1984. Mr. Ludwig earned a B.S. in biology from St. Lawrence University in 1974.
Robert M. Wolfarth has served as our Director of Regulatory Affairs and Quality Assurance since January 2005. From March 2003 to December 2004, Mr. Wolfarth was the Regulatory Affairs Programs Manager at Centerpulse Orthopedics, prior to its acquisition by Zimmer Holdings, Inc., where he was responsible for worldwide regulatory submissions. From March 2000 to March 2003, Mr. Wolfarth was the Regulatory Affairs Manager at Ascension Orthopedics where he was responsible for worldwide regulatory submissions and compliance. He has more than 14 years experience working in the medical devices industry, primarily with respect to large- and small-joint orthopedics, in addition to cardiovascular and medical imaging applications.
Surgeon Advisors
We have engaged surgeon advisors to assist us in designing implants to improve the management of spinal, hip and knee arthritic and trauma disorders. The surgeons are well-known and respected in the spinal and reconstructive orthopedic community. Their works are published in peer reviewed journals, and they frequently serve on the faculty at many society meetings throughout the year. We consult with these surgeons as needed.
The following individuals are our spinal surgeon advisors:
| • | Jean-Jacques Abitbol, M.D. |
| • | Scott D. Boden, M.D. |
| • | Darrel S. Brodke, M.D. |
| • | Andrew T. Dailey, M.D. |
| • | Gregg S. Gurwitz, M.D. |
| • | Alan S. Hilibrand, M.D. |
| • | Carl Lauryssen, M.D. |
| • | Harvinder S. Sandhu, M.D. |
| • | Jeffrey C. Wang, M.D. |
| • | James A. Youssef, M.D. |
The following individuals are our reconstructive surgeon advisors:
| • | B. Sonny Bal, M.D. |
| • | Michael P. Bolognesi, M.D. |
| • | Steven T. Lyons, M.D. |
| • | Rodney L. Plaster, M.D. |
Jean-Jacques Abitbol, M.D. is an advisor for our ceramic spinal implants. Dr. Abitbol is in private practice and is the co-founder of California Spine Group in San Diego, California. He is a former President of the North American Spine Society and the Federation of Spine Associations. Dr. Abitbol has received several awards, including the Cervical Spine Research Society Award for Outstanding Spine Research, the AME Traveling Fellowship Award from the University of Toronto, the Young Investigator Award from the Orthopaedic Research Society, and the Outstanding Spine Research Award from the North American Spine Society AcroMed. He has contributed to more than 27 book chapters, published 36 articles in peer-reviewed journals, and presented 24 abstracts and eight exhibits in the area of spine treatment. He also is an editorial reviewer for the journal Spine.
78
B. Sonny Bal, M.D. is an advisor for our ceramic hip and knee implants. Dr. Bal is an Assistant Professor of Orthopaedic Surgery at the University of Missouri, Columbia, specializing in hip and knee replacement surgery. He also is an Adjunct Professor of Material Sciences at the University of Missouri at Rolla. Dr. Bal received his M.D. from Cornell University and an M.B.A. from Northwestern University, and is a member of the American Academy of Orthopaedic Surgeons and the American Association of Hip and Knee Surgeons.
Scott D. Boden, M.D. is an advisor for our pedicle screw system. Dr. Boden is an internationally recognized orthopaedic surgeon who has served as the Director of the Emory Orthopaedics and Spine Center in Atlanta, Georgia since January 1, 2004. He is a member of many medical societies and editorial boards and serves on the board of several medical societies. Dr. Boden has received numerous honors and awards in his field. Dr. Boden also holds several patents relating to his specialty. Dr. Boden has performed research focusing on three principal areas. One of those areas is cell/molecular biology of osteoblast differentiation, including the study of the mechanism of action of bone growth factors (BMPs) and regional bone gene therapy. The second area in which Dr. Boden has performed significant research focuses on various animal models of spine fixation in an effort to better understand the biology of the healing process and the efficacy of various bone graft substitutes. The third area that Dr. Boden has studied focuses on clinical outcomes relating to spinal disorders, diagnostic imaging and the utilization of health care resources.
Michael P. Bolognesi, M.D. is an advisor for our ceramic hip and knee implants. Dr. Bolognesi is a nationally recognized orthopedic surgeon who specializes in the practice of total joint reconstruction surgery. He also is an Assistant Professor of Surgery at Duke University Medical Center in Durham, North Carolina, where he instructs medical students, residents and fellows in total hip and knee replacement and revision surgery, computer assisted orthopedic surgery, and minimally invasive hip and knee replacement surgery. Dr. Bolognesi received his M.D. from Duke University School of Medicine and served as a resident at Duke University Medical Center. Dr. Bolognesi completed his fellowship training in orthopedic surgery at the University of Utah Medical Center. He has authored many papers on orthopedic surgery and joint reconstruction related topics, presented at national meetings and frequently lectures on the treatment of joint disease. Dr. Bolognesi is an active member of the American Association of Orthopaedic Surgeons, the American Association of Hip and Knee Surgeons and the North Carolina Orthopaedic Association. He has received the Eastern Orthopaedic Association Resident Travel Award, the John Harrelson Chief Resident Teaching Award and the Zimmer Career Development Award.
Darrel S. Brodke, M.D. is an advisor for our ceramic spinal implants. Dr. Brodke is a recognized expert in the field of spine surgery. He also is an Associate Professor in the Department of Orthopedics, University of Utah School of Medicine, and is the Chief of the Spine Service and Medical Director of the University Spine Center. Dr. Brodke is a fellow of the American Academy of Orthopedic Surgery and an active member of the American Medical Association, the North American Spine Society and the Cervical Spine Research Society.
Andrew T. Dailey, M.D. is an advisor for our ceramic spinal implants. Dr. Dailey is Associate Professor in the Department of Neurological Surgery at the University of Utah. Prior to that he was an Associate Professor in the Department of Neurosurgery at the University of Washington and also practiced at Swedish Medical Center in Seattle, Washington, where he specialized in the surgical treatment of cervical, thoracic and lumbar disorders. He completed his residency and fellowship training at the University of Washington. Dr. Dailey is a member of the American Association of Neurological Surgeons, the North America Spine Society and AO North America. Dr. Dailey has authored papers in peer reviewed publications, including Neurosurgery, Journal of Neurosurgery, Journal of Bone and Joint Surgery and Clinical Orthopedics.
Gregg S. Gurwitz, M.D. is an advisor for our ceramic spinal implants. Dr. Gurwitz is an active orthopedic surgeon in San Antonio, Texas, who specializes in treating spinal disease. He completed medical school at Southwestern University in Dallas, Texas, and served as an orthopaedic surgery resident at Vanderbilt University in Nashville, Tennessee. Dr. Gurwitz is an Associate Clinical Professor of Orthopaedic Surgery at the University of Texas Health Science Center at San Antonio. He is a diplomate of the American Board of Orthopaedic Surgery and a fellow of the American Academy of Orthopaedic Surgeons as well as a member of the North American Spine Society. During his 13 years in clinical practice and 10 years of training, Dr. Gurwitz has
79
authored many papers on orthopedic surgery and spinal related topics, presented at national meetings and frequently lectures on the treatment of spinal disease.
Alan S. Hilibrand, M.D. is an advisor for our ceramic spinal implants. Dr. Hilibrand is an Associate Professor of Orthopaedic Surgery and Neurosurgery and is the Director of Orthopedic Medical Education at Jefferson Medical College and The Rothman Institute, both in Philadelphia, Pennsylvania. He is a fellow of the American Academy of Orthopedic Surgeons and a member of the American Orthopaedic Association. Dr. Hilibrand also is the Chairman of the Research Committee of the Cervical Spine Research Society and an active member of the North American Spine Society and the International Society for Study of the Lumbar Spine. Dr. Hilibrand has authored more than 70 peer-reviewed publications and has spoken nationally and internationally on spinal disorders.
Carl Lauryssen, M.D. is an advisor for our ceramic spinal implants. Dr. Lauryssen is a nationally recognized neurosurgeon who specializes in spine treatment and surgery at the Olympia Medical Center in Beverly Hills, California. Prior to that he was an Associate Professor in the Department of Neurological Surgery at Washington University School of Medicine, St. Louis, Missouri and directed the advanced neurosurgical spine program at Barnes-Jewish Hospital in St. Louis, Missouri. Dr. Lauryssen completed his medical school training at the University of Cape Town, South Africa, and served as a resident at University of Calgary, Alberta, Canada and the University of Alabama in Birmingham, Alabama. He has conducted significant research focusing on traumatic spinal cord injury, cervical spondylotic myelopathy, and minimally invasive procedures. Dr. Lauryssen has received the Young Investigator Award from the Neurologic Surgeon’s Society.
Steven T. Lyons, M.D. is an advisor for our ceramic hip and knee implants. Dr. Lyons is a board certified orthopaedist in private practice with the Florida Orthopaedic Institute in Tampa, Florida, specializing in total hip and knee replacement surgery. He received his M.D. from Rush University School of Medicine in Chicago, Illinois, and served as a general surgery internship and orthopaedic surgery resident at Wayne State University Medical Center in Detroit, Michigan. He also was a total joint fellow under Dr. Hofmann at the University of Utah. Dr. Lyons is a diplomate of the National Board of Medical Examiners and is an active member of the American Academy of Orthopaedic Surgeons and the Florida Orthopaedic Society. He also has authored numerous papers on total joint replacement surgery and related topics, has presented at national meetings and frequently lectures on the treatment of joint disease. Dr. Lyons advises and consults with several medical device manufacturers.
Rodney L. Plaster, M.D. is an advisor for our ceramic hip and knee implants. Dr. Plaster is the Director of the Eastern Oklahoma Orthopedic Total Joint Center in Tulsa, Oklahoma and an Assistant Adjunct Professor at the University of Utah Medical Center. He completed his fellowship training at the University of Utah Medical Center. Dr. Plaster has authored many papers on orthopedic surgery and related topics, presented at national meetings and frequently lectures on the treatment of lower joint diseases. Dr. Plaster is a member of the American Academy of Orthopedic Surgeons.
Harvinder S. Sandhu, M.D. is an advisor for our pedical screw system and our bone-plate system. Dr. Sandhu is an Attending Spine Surgeon at the Hospital for Special Surgery and Cornell Medical Center in New York City where he specializes in the surgical treatment of cervical, thoracic and lumbar disorders. He also is the Director of the Spine and Scoliosis Fellowship at the Hospital for Special Surgery. Dr. Sandhu is an active scientist in the Clinical Research division of the institution and teaches medical students, residents and fellows on state-of-the-art techniques in spinal surgery. Prior to joining the Hospital for Special Surgery, Dr. Sandhu was the Chief of the Spine Surgery section of the Department of Orthopaedic Surgery at the University of California, Los Angeles, or UCLA, Medical Center. Dr. Sandhu completed his residency at the State University of New York and fellowship training in spinal surgery at UCLA. He also received an M.B.A. from the Columbia University School of Business. Dr. Sandhu has performed extensive research in the area of bone biology and spinal fixation with an emphasis on methods of stimulating successful fixation. His work led to the commercialization of InFuse Bone Graft Substitute, a highly successful orthobiologic product. Dr. Sandhu has received research awards from the North American Spine Society, the Orthopaedic Research Society and the International Society for the Study
80
of the Lumbar Spine. He has published more than 70 peer-reviewed scientific articles and lectures frequently at national and international symposia. Dr. Sandhu advises and consults with several medical device manufacturers.
Jeffrey C. Wang, M.D. is an advisor for our ceramic spinal implants. Dr. Wang is a nationally recognized expert in the field of spine surgery. He is the Chief of Spine Service of the Department of Orthopedic Surgery at the UCLA School of Medicine; the Director of the Orthopedic Spine Fellowship with the Center for Health Sciences at UCLA; a co-Director of the UCLA Spine Center; and Chief of the Spine Service at the West Los Angeles Veterans Administration, California. He is also an Associate Professor of Orthopaedics and Neurosurgery at the UCLA School of Medicine, and serves on the board of directors of the North American Spine Society and on the Editorial Committee of SpineLine magazine.
James A. Youssef, M.D. is an advisor for our ceramic spinal implants. Dr. Youssef is a recognized expert in the field of spine surgery. He received his M.D. at University of California, Irvine School of Medicine in 1991. Dr. Youssef completed his internship in general surgery at Oregon Health Sciences University in 1992 and his residency in orthopedic surgery at Dartmouth-Hitchcock Medical Center in 1996. He completed his Spine fellowship at the University of California, Davis Medical Center in 1997. Dr. Youssef is the co-founder of SpineColorado and a senior partner in Durango Orthopedic Assoc., P.C. He also is a fellow of the American Academy of Orthopedic Surgery and an active member of the American Medical Association, the North American Spine Society and the Orthopedic Trauma Association.
Board Composition
Our amended and restated certificate of incorporation and amended and restated bylaws provide that the authorized number of directors may be changed only by resolution of the board of directors. Seven directors are currently authorized. In accordance with our amended and restated certificate of incorporation, immediately upon the closing of this offering, our board of directors will be divided into three classes with staggered three-year terms. At each annual meeting of stockholders following this offering, the successors to the directors whose terms then expire will be elected to serve until the third annual meeting following the election. At the closing of this offering, our directors will be divided among the three classes as follows:
| • | The Class I directors will be Aaron A. Hofmann, M.D., Ashok C. Khandkar, Ph.D. and Rohit Patel, and their terms will expire at the annual meeting of stockholders to be held in 2008; |
| • | The Class II directors will be Lawrence D. Dorr, M.D. and Gregg R. Honigblum, and their terms will expire at the annual meeting of stockholders to be held in 2009; and |
| • | The Class III directors will be Bradford S. Goodwin and Max Link, Ph.D., and their terms will expire at the annual meeting of stockholders to be held in 2010. |
Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors.
Director Independence
Our board of directors has reviewed the materiality of any relationship between us and each of our directors, either directly or indirectly. Based on this review, the board has determined that Max Link, Ph.D., Aaron A. Hofmann, M.D., Lawrence D. Dorr, M.D., Rohit Patel and Bradford S. Goodwin are “independent directors” as defined by the SEC and NASDAQ. The rules of The NASDAQ Global Market require that a majority of the board of directors of a listed company consist of independent directors, as defined by the rules of The NASDAQ Global Market. We currently have a board of directors consisting of a majority of independent directors.
81
Committees of the Board of Directors
Our board of directors has an audit committee, a compensation committee and a nominating and corporate governance committee, each of which has the composition and responsibilities described below. The rules of The NASDAQ Global Market require that the audit committee consist of at least three members of our board of directors, each of whom must be independent, as established under the rules of The NASDAQ Global Market and the SEC.
Audit Committee
Our audit committee is composed of Bradford S. Goodwin (Chairman), Max Link, Ph.D. and Rohit Patel, each of whom is independent within the meaning of the rules of the SEC and the listing standards of The NASDAQ Global Market. Our board of directors has appointed Bradford S. Goodwin as our audit committee financial expert. All of our independent auditors and management periodically meet privately with our audit committee. Our audit committee is authorized to:
| • | approve and retain the independent auditors to conduct the annual audit of our books and records; |
| • | review the proposed scope and results of the audit; |
| • | review and pre-approve the independent auditor’s audit and non-audit services rendered; |
| • | approve the audit fees to be paid; |
| • | review accounting and financial controls with the independent auditors and our financial and accounting staff; |
| • | review and approve transactions between us and our directors, officers and affiliates; |
| • | recognize and prevent prohibited non-audit services; |
| • | establish procedures for complaints received by us regarding accounting matters; |
| • | oversee internal audit functions; and |
| • | prepare the report of the audit committee that SEC rules require to be included in our annual meeting proxy statement. |
Compensation Committee
Our compensation committee is composed of Rohit Patel (Chairman), and Lawrence D. Dorr, M.D., and Bradford S. Goodwin, each of whom is independent within the meaning of the rules of the SEC and The NASDAQ Global Market. Gregg R. Honigblum served as a member of our compensation committee from December 2006 until March 2007. Our compensation committee is authorized to:
| • | review and recommend the compensation arrangements for management, including the compensation for our president and chief executive officer; |
| • | establish and review general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; |
| • | administer our stock incentive plan; and |
| • | prepare the report of the compensation committee that SEC rules require to be included in our annual meeting proxy statement. |
82
Nominating and Governance Committee
Our nominating and governance committee is composed of Rohit Patel (Chairman) and Max Link, Ph.D., each of whom is independent within the meaning of the rules of the SEC and The NASDAQ Global Market. Our nominating and governance committee is authorized to:
| • | identify and nominate members of the board of directors; |
| • | develop and recommend to the board of directors a set of corporate governance principles applicable to our company; and |
| • | oversee the evaluation of the board of directors and management. |
Compensation Committee Interlocks and Insider Participation
Our compensation committee is composed of Rohit Patel, Lawrence D. Dorr, M.D., Bradford S. Goodwin and Gregg R. Honigblum served as a member of our compensation committee from December 2006 until March 2007. No member of our compensation committee has at any time been an employee of ours. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Each of Mr. Patel, Dr. Dorr and Mr. Honigblum have participated in transactions with us since January 2004. For a detailed description of these transactions, see “Certain Relationships and Related Person Transactions” on page 105.
83
COMPENSATION DISCUSSION AND ANALYSIS
Overview
The following Compensation Discussion and Analysis describes material aspects of our executive compensation policies and decisions as they relate to the compensation of our named executive officers. In this section of this prospectus we discuss and analyze the objectives of our compensation program and what our compensation program is designed to reward. We also discuss and analyze the material elements of our compensation program, why we choose to pay each element, how we determine the amount of each element, and how each element of compensation fits into our overall compensation objectives. In addition, we describe actions regarding compensation taken before and after 2006 to the extent these actions enhance the understanding of our 2006 executive compensation program.
As our business has developed, and with the guidance and input of our compensation committee, we have developed and implemented compensation policies, plans and programs over the past few years that we believe help us achieve the goals and objectives of our compensation program. Our board of directors appoints the members of our compensation committee and delegates to that committee oversight of the administration of our executive compensation. The compensation committee assesses executive compensation by applying the following principles: the level of executive compensation should depend upon both corporate performance and individual performance; the interests of our executives should be closely aligned with those of our stockholders through equity-based compensation; and compensation should be commensurate with our stage of development. Our compensation committee makes recommendations to our board of directors based upon recommendations provided to the compensation committee by our Chief Executive Officer as to annual base salary increases and annual stock option awards for the other executive officers. Recommendations for annual base salary increases and annual stock option awards for Dr. Khandkar are determined by the compensation committee without any input from Dr. Khandkar. Our board of directors considers and ultimately acts on the recommendations of the compensation committee regarding the compensation of our executive officers, including our Chief Executive Officer. Our board of directors has authority to follow, modify or reject these recommendations. Our Chief Executive Officer does not vote on matters concerning his compensation. Such matters are considered and acted on by a majority of independent directors.
Executive Compensation Program Objectives and Philosophy
The primary objectives of our executive compensation program are to:
| • | attract, motivate and retain talented and dedicated executive officers to a development-stage company; |
| • | provide our executive officers with both cash and equity incentives to promote strong performance; |
| • | provide our executive officers with long-term incentives, in the form of stock options, in order to align the interests of our executive officers with those of our stockholders; and |
| • | provide continuity during our development stage. |
Either in the fourth quarter of the prior fiscal year or in the first quarter of the then current fiscal year, the compensation committee, in conjunction with management, sets recommendations for base salaries to be paid and stock option awards to be granted to all employees, including our executive officers. In making annual recommendations for base salaries and stock option awards, the compensation committee reviews our overall corporate position and product development progress near the end of the fiscal year and the individual performance of each executive officer.
In 2006, our Chief Executive Officer made recommendations to our compensation committee as to individual performance goals for all executive officers other than himself. These individual performance goals relate to specific corporate functions for which each executive officer is either principally responsible or for which his performance significantly influences the outcome. In 2006, our compensation committee formulated these individual performance goals taking into consideration recommendations made by our Chief Executive
84
Officer. Our board of directors ultimately approved the recommendations of our compensation committee as to the individual performance goals for all executive officers. The individual performance goals recommended by our Chief Executive Officer were substantially similar to the performance goals ultimately approved by our board of directors. However, we are unable to elaborate on specific differences between the performance goals initially recommended by the Chief Executive Officer and the goals ultimately recommended by the compensation committee and approved by the board of directors, because the Chief Executive Officer’s recommendations were conveyed to the compensation committee during oral discussions, and neither the committee nor the board maintained a system for retaining the Chief Executive Officer’s recommendations or the committee’s specific modifications of those recommended goals in writing with enough specificity to track modifications over time. In addition, due to significant changes in the composition of the compensation committee since late-2005 and early-2006, when these performance goals were established, there is insufficient institutional memory to reconstruct the differences with specificity since the sole continuing member of the committee from that period of time is unable to do so. Starting with fiscal year 2007, the compensation committee and board of directors have retained information about the performance goal recommendations made to the compensation committee and modifications made by the committee and/or the board of directors to those recommendations in establishing individual executives’ performance goals.
Beginning in fiscal year 2007, following a change in our internal reporting structure initiated by our board of directors, our Chief Executive Officer made recommendations to our compensation committee as to the individual performance goals for our President and our Vice President of Finance and Chief Financial Officer, each of whom reports directly to our Chief Executive Officer. Our President made recommendations to our compensation committee as to the individual performance goals for all other executive officers. Our compensation committee formulated the individual performance goals for our President and our Vice President of Finance and Chief Financial Officer taking into consideration the recommendations made by our Chief Executive Officer. In addition, our compensation committee formulated the individual performance goals for all other executive officers taking into consideration the recommendations made by our President. Following consideration of the recommendations of the compensation committee, our board of directors considered and ultimately made final decisions with regard to the setting of individual performance goals for all of our executive officers. The individual performance goals recommended by our Chief Executive Officer and President were substantially similar to the performance goals ultimately approved by our board of directors. However, our compensation committee and board of directors in each case modified the recommended goals by reducing the total number of specific goals and tying them more closely to critical milestones in the process of developing, manufacturing, obtaining marketing clearance for, and launching our lead product candidates. In this way, the committee and board sought to eliminate goals viewed as less significant implementation steps and focus individual executives’ performance goals on objectives viewed as important measures of progress toward our success in commercializing our first products. These performance goals were incorporated into the accelerated vesting provisions of stock options recently granted to our President, Vice President of Manufacturing and Research, Vice President of Finance and Chief Financial Officer, and Vice President of Marketing, and are described below.
The performance goals for our executive officers for 2006 included the following:
Ashok C. Khandkar, Ph.D.—lead the executive team in planning and executing corporate, financial, strategic business plans and objectives; manage our operations; manage the development of our commercial scale manufacturing facilities; further our growth and advance our product development process.
Eugene B. Jones—participate with the Chief Executive Officer and the executive team in planning and executing corporate, financial, strategic business plans and objectives; confirm with the Chief Executive Officer and the executive team that our financial capital needs are adequately met; provide adequate cost controls and timely reporting to effectively manage our finances; oversee human resources and administration functions; and participate in decision making with regard to corporate transactions.
Warionex (“Jose”) Belen—participate with the Chief Executive Officer and the executive team in planning and executing corporate, product development and strategic business plans, including intellectual property and
85
our overall company objectives; confirm with the Chief Executive Officer and the executive team that our product development and intellectual property objectives are adequately met; and provide adequate budgetary controls and timely reporting to effectively manage our product development.
Bryan J. McEntire—participate with the Chief Executive Officer and the executive team in planning and executing corporate, financial, strategic business plans and objectives; meet manufacturing capital equipment budget and spending plan for 2006; retain ISO 13485 certification; develop manufacturing processes and building out our manufacturing facility.
Cameron G. Rouns—participate with the Chief Executive Officer and the executive team in planning and executing corporate, financial, strategic business plans and objectives; confirm with the Chief Executive Officer and the executive team that our sales and marketing objectives are adequately met; and provide adequate budgetary controls and timely reporting to effectively manage our sales and marketing.
In 2006, the compensation committee recommended, and our board of directors approved, an opportunity for our Chief Executive Officer to receive a cash bonus, the payment of which was dependent upon our achieving specified corporate goals in 2006 that the board of directors believed should be achieved that fiscal year. Our Chief Executive Officer was the only employee eligible to receive a bonus pursuant to this opportunity. If the corporate goals had been achieved, our Chief Executive Officer would have received a cash bonus of up to a maximum of 25% of his 2006 base salary. Our board of directors approved our compensation committee’s recommendations as to the size of the bonus opportunity and the formulation of the corporate goals related to this bonus opportunity without modification. The extent to which our Chief Executive Officer achieved these specified corporate goals impacted only this cash bonus opportunity and had no influence on any other elements of our Chief Executive Officer’s compensation in 2006.
The specified performance goals for 2006 approved by our board of directors related to this cash bonus opportunity for our Chief Executive Officer included the completion of an initial public offering, launch of certain spinal product candidates, achievement of targets for specified product revenues and net loss for 2006 and a specified amount of our cash balance at fiscal year end, establishment of silicon nitride manufacturing capabilities with the company and an increase in the number of qualified suppliers of silicon nitride.
Our board of directors decided to use the cash bonus opportunity as an incentive for our Chief Executive Officer because they believed that it constituted an appropriate balancing of the need to conserve company resources for our key product development efforts with the incentive impact of a cash bonus. Mindful of the need to conserve our cash resources, the board believed that a broad-based cash bonus program in which other executives were eligible to participate would not be appropriate for a company of our size or our stage of development. At the same time, our board of directors believed that it was appropriate to institute a limited cash bonus opportunity for our Chief Executive Officer because he was the executive in the best position to manage our business towards achievement of the specified goals. Moreover, our board of directors, at that time, believed that a grant of stock options to our Chief Executive Officer might not constitute as effective an incentive as a cash bonus opportunity given Dr. Khandkar’s significant equity stake in our company. In contrast, our board believed that other executives, with much smaller equity positions, would find stock option awards to be more attractive performance incentives.
A cash bonus opportunity for our Chief Executive Officer was not renewed in 2007. Our board of directors determined that in light of the anticipated increase in our cash burn rate to support our key product development efforts, it was important to conserve cash for those priorities during 2007. The board determined therefore to limit the company’s incentive programs to equity-based incentives consisting of stock option awards.
Beginning with fiscal year 2007, the compensation committee recommended, and our board of directors approved, the grant of stock options to our President, Vice President of Finance and Chief Financial Officer, Vice President of Manufacturing and Research, and Vice President of Marketing that include an accelerated vesting feature tied to the achievement of certain individual and corporate performance goals. The corporate goals for these named executive officers relate to development milestones for our lead product candidates, and the
86
individual goals are related to one or more corporate functions for which each such executive is principally responsible. The specific targets associated with each executive officer’s performance goals are sensitive commercial information that, if publicly disclosed, could hinder our ability to effectively compete against our competitors, or put us at a significant competitive disadvantage in negotiations with third parties. Barring unforeseen circumstances, we expect that it is reasonably likely that our executive officers will achieve their individual targets set in 2007.
Our board of directors did not grant stock options to our Chief Executive Officer that include an accelerated vesting feature tied to achievement of specified performance goals for 2007. This decision was based on the recommendation of our compensation committee, in recognition of Dr. Khandkar’s status as a significant stockholder of our company. For purposes of this particular set of option grants, the compensation committee believed that the incentive impact of a relatively sizable stock option grant for Dr. Khandkar would not be as great as the incentive impact on other executive officers who have substantially smaller equity stakes in our company and for whom accelerated vesting would therefore provide a significant incentive. In addition, these stock options were granted at a time when the there were a limited number of shares available in our 2003 Stock Option Plan, and therefore priority was given to executive officers with less significant equity positions in our company. However, Dr. Khandkar remains eligible to received stock-based awards under our stock-based compensation plans.
The 2007 performance goals for our President, Vice President of Manufacturing and Research, Vice President of Finance and Chief Financial Officer, and Vice President of Marketing include the following:
Warionex (“Jose”) Belen—preparation of filings for submission to the FDA for our lead spinal products; execution of sales agent agreements for our spinal products; completion of the design of our reconstructive product candidates; and the first commercial sale of one of our lead spinal products.
Bryan J. McEntire—completion of installation and operational qualifications for the manufacture of our lead spinal products; manufacture of sufficient quantities of our lead spinal products for testing; and the first commercial sale of one of our lead spinal products.
Reyn E. Gallacher—completion of an initial public offering and the first commercial sale of one of our lead spinal products.
Kenneth W. Ludwig, Jr.—execution of sales agent agreements for our spinal products; completion of documentation for product training, education and sales materials of our lead product candidates; and the first commercial sale of one of our lead spinal products.
If an executive’s individual goals are achieved, then a specified portion of the executive’s option immediately vests, and if all of the executive’s individual goals are achieved and all of the corporate goals are achieved, then the executive’s option will immediately become fully vested. If the corporate goals and the executive’s individual goals are not met, then these stock options vest over a four-year period following the date they were granted.
In 2007, the compensation committee also recommended, and our board of directors approved, performance goals for our Chief Executive Officer. These performance goals are directly tied to our board of directors’ determinations of whether our Chief Executive Officer may receive an annual base salary adjustment and the number of stock options, if any, he may receive. These performance goals include the setting and execution of our overall corporate strategies, completion of our initial public offering, and management and supervision of our President and Vice President of Finance and Chief Financial Officer.
In addition, in 2007, our board of directors approved a change in our management organization from a two-tier to three-tier structure, thereby changing the performance goals of our Chief Executive Officer and President. The performance goals for all other executive officers are the same as those goals in 2006. The performance goals for our President include working with the Chief Executive Officer in the setting and execution of our overall corporate strategies, and the management and supervision of all other executive officers and department heads, including manufacturing, sales and marketing, product development and regulatory matters.
87
With the exception of our Chief Executive Officer, the written evaluation of our executive officers is performed in January of each year. Our Chief Executive Officer’s performance evaluation, which generally is conducted by the compensation committee in December of each year, influences his base salary adjustments and stock awards, if any. Our Chief Executive Officer prepares written evaluations of the other executive officers. Both the Chief Executive Officer and other executive officer then meet to discuss the executive officer’s evaluation and the performance of that executive officer relative to established goals. Supervisors are responsible for completing a written evaluation of the performance of all employees who report directly to them. Individual goals for non-executive employees for the following year are proposed and agreed to jointly by the individual non-executive employee and his or her direct supervisor. The following year, the supervisor and non-executive employee meet to discuss the evaluation and his or her performance relative to these established goals. This process culminates in a recommendation by the Chief Executive Officer and Chief Financial Officer to the compensation committee for annual executive officer and non-executive employee base salary increases and annual stock option awards, if any. The compensation committee determines the award of any base salary increases and stock option grants to our executive officers. Base salary increases for individual employees, including our executive officers, are allocated based on individual merit and other considerations from a “pool” equal to a specified percentage of aggregate current base salaries. These recommendations are then reviewed by the compensation committee and recommended to the board of directors for approval.
The Elements of Our Compensation Program
The principal elements of our executive compensation program are base salary, long-term equity incentives in the form of stock options to purchase our common stock, post-termination severance, and acceleration of stock option vesting upon certain termination and/or change in control events. We do not currently have an annual cash bonus program.
Base Salary
Base salary is used to compensate our executive officers based on the breadth of their experience, skills, knowledge and responsibilities, taking into account our overall financial position and product development progress. Salaries for our executive officers are reviewed by our Chief Executive Officer and the compensation committee on an annual basis, as well as at the time of promotion or times of other significant changes in responsibilities. The recommended base salaries for our executive officers for 2006 were determined by the compensation committee after reviewing a number of factors, including:
| • | the responsibilities associated with the position held by each of our executive officers and where that position fits within our overall corporate structure; |
| • | the seniority of the individual executive’s position; |
| • | the base salary level of each executive officer in prior years; |
| • | our overall financial position and product development progress; and |
| • | for executive officers other than the Chief Executive Officer, recommendations made by the Chief Executive Officer. |
The compensation committee does not assign relative weights or rankings to these factors, but instead makes a subjective determination taking into account all of these factors.
In December 2005, the compensation committee recommended and our board of directors approved, a 7% merit increase in the base salary of our Chief Executive Officer effective as of December 1, 2005. In December 2006, the compensation committee recommended, and our board approved, a 5% merit increase in the base salary of our Chief Executive Officer effective as of January 2007. The compensation committee based this increase on our overall financial position; his ongoing contributions towards our future potential private and public financings, the completion of our intellectual property review, management of the development of our
88
commercial scale manufacturing facilities; and his ability to further our growth and advanced product development progress.
In December 2006, our Vice President of Products, Jose Belen, was promoted to the position of President. In recognition of the fact that Mr. Belen was assuming frontline responsibility for day-to-day operations of the company, the compensation committee recommended that his annual base salary be set at an amount almost equal to that of Dr. Khandkar, whose role formerly encompassed that major responsibility. Dr. Khandkar’s annual base salary at the time was $200,000, and accordingly, the board of directors determined to set Mr. Belen’s annual base salary at $195,000. In January 2007, our board of directors also voted to promote Reyn Gallacher, our former Controller, to the position of Vice President of Finance, and he was further promoted to the position of Chief Financial Officer in March 2007. In connection with his appointment as Chief Financial Officer, his annual base salary was increased to $150,000 in recognition of his substantially increased responsibilities with respect to internal controls and financial reporting and his anticipated contributions to our future financing efforts.
In connection with our management restructuring, Dr. Khandkar was re-appointed as our Chief Executive Officer with overall responsibility for strategic planning and direction, as well as direct responsibility for leading our financing initiatives and overseeing our relationship with investors. Subsequent to this restructuring, the compensation committee recommended in March 2007 that Dr. Khandkar’s annual base salary be increased to $230,000, in recognition of his achievements in positioning the company to pursue additional financings, including overseeing the completion of our intellectual property review, coordinating with the company’s placement agent for the successful completion of our Series D financing and helping to lead a strategic re-focusing of our product development efforts. The board of directors was considering a number of compensation matters in March 2007 (including the performance-based stock options described above) and decided to delay action on the compensation committee’s recommendation with respect to Dr. Khandkar’s salary increase until all these compensation matters were resolved. The board of directors acted on this recommendation in early-May 2007. In light of the board of directors’ decision to delay action on the compensation committee’s recommendation, the board of directors believed it was appropriate to make the increase in annual base salary for the Dr. Khandkar retroactive to March 2007. See “—Executive Compensation—Employment Arrangements with Ashok C. Khandkar, Ph.D.—Offer Letters” below.
Long-Term Incentives
We provide the opportunity for our executive officers to earn long-term equity incentive awards. Long-term equity incentive awards provide our executives with the incentive to continue their employment with us for longer periods of time, which in turn, provides us with greater continuity during our growth stage. In 2006, our long-term equity incentive program consisted solely of grants of stock options. Also, these grants of stock are less costly to us in the short-term than cash compensation.
Initial Stock Options
Executive officers who join us are awarded initial stock option grants. These grants have an exercise price equal to the fair market value of our common stock on the grant date and a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. The amount of the initial stock option award is determined based on the executive’s position and our overall financial position and product development progress. The initial stock option grants are intended to provide the executive, promptly upon joining us, with an incentive to build value in our company over an extended period of time. The amount of the initial stock option award is also determined in light of the executive’s base salary and other compensation to ensure that the executive’s total compensation is in line with our overall compensation philosophy.
Annual Stock Options
It is the intention of the compensation committee to award long-term equity incentives to executives on an annual basis as part of our overall performance review process, although more frequent awards may be made at
89
the recommendation of the compensation committee and upon approval by the board of directors, such as in the case of promotions or newly hired executives. The size of individual executive stock option grants is influenced by several factors, including our overall financial position and product development progress, the responsibilities of the individual executive officer, the executive officer’s past performance, anticipated future contributions, prior option grants, and the executive officer’s total cash compensation. Our equity incentive awards granted to executive officers in 2006 were recommended by our compensation committee and approved by our board of directors based on these factors and were intended to provide management with a strong incentive to maximize corporate performance and the creation of stockholder value over the long term. We granted stock options to all of our executive officers at exercise prices equal to the fair market value of our common stock on the date of grant. All of these option grants are subject to a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. We believe that these time-based vesting provisions reward the longevity and commitment of our executive officers. We have used stock options as our form of equity compensation because, among other things, stock options result in less immediate dilution of existing stockholders’ interest and, prior to our adoption of SFAS 123(R), resulted in less compensation expense for us relative to other types of equity awards.
In December 2006, the compensation committee, as part of its annual review process and based upon our overall financial position and product development progress, recommended, as it did in 2005, that our board of directors grant stock options to our executive officers in order to reward the contributions they made towards the achievement of our corporate goals and to more closely align our executives’ ownership interests with the long-term interests of our stockholders.
In May 2007, the compensation committee recommended, and our board of directors approved, the grant of stock options to our President, Vice President of Finance and Chief Financial Officer, Vice President of Manufacturing and Vice President of Marketing that include an accelerated vesting feature tied to the achievement of certain individual and corporate performance goals. See “—Executive Compensation—Offer Letters” below.
Other Compensation
We maintain broad-based benefits that are provided to all employees, including health insurance, of which we pay 50% of the premiums, life insurance, long-term disability insurance, and a 401(k) plan. Effective upon the completion of our first full payroll period following July 1, 2007, we plan to begin matching the contributions of our employees who participate in our 401(k) plan as follows: a match of 100% on the first 3% of compensation contributed by a plan participant and a match of 50% on amounts above 3%, up to a limit of 5%, of compensation contributed by a plan participant.
Severance and Change in Control Benefits
We provide employment protections for our named executive officers and our Vice President of Marketing by including severance benefits and change in control provisions in their severance agreements. We provide these protections in order to attract and retain highly skilled and experienced executive officers, as well as to align the interests of our executives with those of our stockholders.
If, within one year following a change in control, our Chief Executive Officer is terminated by us other than for cause, or resigns for good reason, he is entitled to receive a lump sum severance payment of an amount equal to three times his highest annual base salary and bonus during the preceding three-year period, including the year of such termination. Under the same circumstances, our other named executives are entitled to receive a lump sum severance payment of an amount equal to two times their highest annual base salary and bonus during the preceding three-year period, including the year of termination. If it is determined that the amounts payable to each executive officer under his severance agreement, when considered together with any other payments payable to him in connection with a change in control of Amedica, cause these payments to be treated as excess parachute payments under Section 280G of the U.S. Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, then we will be required to
90
make an additional “gross up” payment in order to pay for any additional tax imposed on him pursuant to Section 4999 of the Internal Revenue Code. We believe that the increased difficulty of finding comparable employment opportunities at the level of chief executive officer requires us to provide longer terms for severance payments in order to attract and retain highly skilled and experienced individuals for this position. In addition, within one year following a change in control, certain provisions of our executive officers’ severance agreements allow for acceleration of equity awards in the event the executive is terminated without cause or the executive terminates their employment for good reason. We believe that this equity vesting acceleration mechanism provides an incentive for our executive officers to achieve corporate and individual goals and rewards them for their part in increasing our value, while contemporaneously incentivizing them to maintain their employment after a friendly change in control.
Our severance and change in control provisions for our executive officers and the definitions of cause, good reason, and change in control are summarized in “—Potential Payments Upon Termination or Change in Control” below.
Conclusion
Our compensation policies are designed and are continually being developed to retain and motivate our executives and to reward them for outstanding individual and corporate performance.
91
Summary Compensation Table
The following table shows the total compensation paid or accrued during the fiscal year ended December 31, 2006 to (1) our Chief Executive Officer, (2) our President, (3) our current Vice President, Finance and Chief Financial Officer, (4) our former Vice President, Finance and Chief Financial Officer, and (5) our only other executive officer who earned more than $100,000 during the fiscal year ended December 31, 2006. The table also includes an additional executive officer who earned more than $100,000 during the 2006 fiscal year but who was not serving as an executive officer of Amedica at December 31, 2006.
| Name and Principal Position at May 1, 2007 |
Year | Salary | Option Awards(1) |
All Other Compensation |
Total | ||||||||||
| Ashok C. Khandkar, Ph.D. Chief Executive Officer |
2006 | $ | 190,372 | $ | 10,874 | $ | — | $ | 201,246 | ||||||
| Warionex (“Jose”) Belen President |
2006 | 155,262 |
|
4,708 |
— | 159,970 | |||||||||
| Bryan J. McEntire Vice President, Manufacturing and Research |
2006 | 155,758 |
|
9,225 |
— | 164,983 | |||||||||
| Reyn E. Gallacher Vice President, Finance, Chief Financial Officer and Assistant Secretary(2) |
2006 | 105,923 |
|
7,558 |
— | 113,481 | |||||||||
| Eugene B. Jones Former Vice President, Finance and Chief Financial Officer(3) |
2006 | 155,446 |
|
9,388 |
95,489 | (4) | 260,323 | ||||||||
| Cameron G. Rouns Former Vice President, Sales and Marketing(5) |
2006 | 135,661 |
|
3,586 |
— | 139,247 | |||||||||
| (1) | The dollar amounts in this column represent the compensation cost for the year ended December 31, 2006 of stock option awards granted in and prior to 2006. These amounts have been calculated in accordance with FASB Statement No. 123 (revised), “Share-Based Payment,” or SFAS No. 123R, using the Black-Scholes Valuation model. See Notes 1 and 7 to our financial statements included elsewhere in this prospectus for details as to assumptions used to determine the fair value of the option awards. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates—Stock-Based Compensation.” |
| (2) | Mr. Gallacher served as our Controller during the fiscal year ended December 31, 2006 and became our Vice President, Finance on January 6, 2007, and our Chief Financial Officer and Assistant Secretary in March 2007. |
| (3) | Mr. Jones resigned effective January 5, 2007. |
| (4) | Represents a lump sum severance payment in connection with Mr. Jones’ separation of employment. |
| (5) | Mr. Rouns resigned effective December 1, 2006. |
92
Grants Of Plan-Based Awards
The following table shows information regarding grants of plan-based awards that we made during the fiscal year ended December 31, 2006 to each of the executive officers named in the Summary Compensation Table.
| Name |
Grant Date | All Other Option Awards: Number of Securities Underlying Options |
Exercise or Base Price of Option Awards (per share) |
Grant Date Fair Value of Stock and Option Awards(1) | |||||||
| Ashok C. Khandkar, Ph.D. |
2/12/2006 | (2) | 10,472 | $ | 3.82 | $ | 3.13 | ||||
| Chief Executive Officer |
12/11/2006 |
(3) |
10,472 | 3.82 | 2.90 | ||||||
| Warionex (“Jose”) Belen |
2/12/2006 | (2) | 1,571 | 3.82 | 3.13 | ||||||
| President |
12/11/2006 | (3) | 7,854 |
|
3.82 |
2.90 | |||||
| Bryan J. McEntire |
2/12/2006 | (2) | 7,854 |
|
3.82 |
3.13 | |||||
| Vice President, Manufacturing and Research |
12/11/2006 | (3) | 7,854 |
|
3.82 |
2.90 | |||||
| Reyn E. Gallacher |
2/12/2006 | (4) | 10,472 |
|
3.82 |
3.13 | |||||
| Vice President, Finance, Chief Financial |
12/11/2006 | (3) | 7,854 | 3.82 | 2.90 | ||||||
| Officer and Assistant Secretary |
|||||||||||
| Eugene B. Jones |
2/12/2006 | (2) | 7,854 | 3.82 | 3.13 | ||||||
| Former Vice President, Finance and Chief Financial Officer |
|||||||||||
| Cameron G. Rouns |
2/12/2006 | (2) | 1,571 | 3.82 | 3.13 | ||||||
| Former Vice President, Sales and Marketing |
|||||||||||
| (1) | See Notes 1 and 7 to our financial statements included elsewhere in this prospectus for details as to assumptions used to determine the fair value of the option awards. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates—Stock-Based Compensation.” |
| (2) | Represents an annual stock option granted for performance during the fiscal year ended December 31, 2005. |
| (3) | Represents an annual stock option granted for performance during the fiscal year ended December 31, 2006. |
| (4) | Represents a stock option granted to Mr. Gallacher when he joined us on January 6, 2006. |
Employment Arrangements with Ashok C. Khandkar, Ph.D.
We do not have a written employment agreement with Ashok C. Khandkar, Ph.D., our Chief Executive Officer, and he is employed by us on an at-will basis. As of December 31, 2006, Dr. Khandkar’s base salary was $190,000. In addition, Dr. Khandkar is eligible to receive annual stock option grants based upon our overall financial position and product development progress, and the attainment of specified performance goals recommended by the compensation committee and approved by our board of directors. Based upon our overall financial position and product development process for the fiscal year ended December 31, 2005, Dr. Khandkar received an option to purchase 10,472 shares of our common stock at an exercise price of $3.82 per share on February 12, 2006. For the fiscal year ending December 31, 2006, Dr. Khandkar was eligible to receive a cash bonus of up to 25% of his base salary based upon the achievement of certain performance goals established by our board of directors. Because we did not launch any of our product candidates in 2006 and we did not complete an initial public offering, Dr. Khandkar was not paid a cash bonus for 2006. For the fiscal year ended December 31, 2006, based on our overall financial position; his ongoing contributions towards our future potential private and public financings, management of the development of our commercial scale manufacturing facilities; and his ability to further our growth and advance product development progress, Dr. Khandkar received a 5% merit base salary increase, which increased his salary to $200,000 effective January 1, 2007, and he
93
received an option to purchase 10,472 shares of our common stock at an exercise price of $3.82 per share. The option is subject to a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. In May 2007, in recognition of his achievements in positioning the company to pursue additional financings, including overseeing the completion of our intellectual property review, coordinating with the company’s placement agent for the successful completion of our Series D financing and helping to lead a strategic re-focusing of the company’s product development efforts, the compensation committee recommended, and our board of directors approved, an increase in Dr. Khandkar’s base salary to $230,000 per year retroactive to March 2007.
Dr. Khandkar has entered into a confidentiality and assignment of inventions agreement pursuant to which he has agreed to maintain the confidentiality of our business information and assign his past and present inventions to us. In addition, Dr. Khandkar is entitled to certain benefits in connection with a termination of his employment upon a change in control discussed below under “—Potential Payments Upon Termination or Change in Control.”
Offer Letters
We do not have written employment agreements with any of our other named executive officers and each of our executive officers is employed by us on an at-will basis. However, certain elements of the executive officers’ compensation and other employment arrangements are set forth in letter agreements that we executed with the executive officers at the time their employment with us commenced. These letter agreements provide, among other things, the executive officer’s initial annual base salary and initial stock option grant. As a condition to their employment, each executive officer has entered into a confidentiality and assignment of inventions agreement pursuant to which each officer has agreed to maintain the confidentiality of our business information and assign inventions to us. The letter agreements are further described below. Since the date of each of the letter agreements entered into with our executive officers, the compensation paid to each has been increased and additional stock options have been granted.
Warionex (“Jose”) Belen, President. Pursuant to a letter agreement dated May 31, 2005 between us and Mr. Belen, we agreed to employ Mr. Belen as our Vice President of Products beginning on May 31, 2005. In December 2006, Mr. Belen began serving as our President and, in recognition of the fact that Mr. Belen was assuming frontline responsibility for day-to-day operations of the company, his base salary was increased to $195,000 effective January 1, 2007. Based upon our overall financial position; our product development progress; and his contributions towards planning and executing product development plans, and providing adequate budgetary controls and timely reporting to effectively manage our product development for the fiscal year ended December 31, 2005, Mr. Belen received an option to purchase 1,571 shares of our common stock at an exercise price of $3.82 per share on February 12, 2006.
Based upon our overall financial position; our product development progress; and his contributions towards planning and executing product development plans, and providing adequate budgetary controls and timely reporting to effectively manage our product development for the fiscal year ended December 31, 2006, and in connection with his promotion to our President, Mr. Belen also received an option to purchase 7,854 shares of our common stock at an exercise price of $3.82 per share. The option is subject to a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. In May 2007, the compensation committee recommended, and our board of directors approved, the grant of 26,179 stock options to Mr. Belen that include an accelerated vesting feature tied to the achievement of certain individual and corporate performance goals for 2007 and 2008. Mr. Belen is entitled to certain benefits in connection with a termination of his employment upon a change in control discussed below under “—Potential Payments Upon Termination or Change in Control.”
Bryan J. McEntire, Vice President, Manufacturing and Research. Pursuant to a letter agreement dated May 29, 2004 between us and Mr. McEntire, we agreed to employ Mr. McEntire as our Vice President of Manufacturing beginning August 1, 2004. He became our Vice President of Research in December 2006. In
94
order to more closely align the salaries of our executive officers relative to their individual position and in recognition of Mr. McEntire’s ongoing contributions to our growth and development, his base salary was increased to $190,000 effective January 1, 2007. Based upon our overall financial position; our product development progress; and his contributions towards meeting manufacturing our capital equipment budget and spending plan for 2005, retaining ISO 13845 certification, and developing manufacturing processes for the fiscal year ended December 31, 2005, Mr. McEntire received an option to purchase 7,854 shares of our common stock at an exercise price of $3.82 per share on February 12, 2006.
Based upon our overall financial position; our product development progress; and his contributions towards meeting manufacturing our capital equipment budget and spending plan for 2006, retaining ISO 13845 certification, and developing manufacturing processes and building out our manufacturing facility for the fiscal year ended December 31, 2006, Mr. McEntire also received an option to purchase 7,854 shares of our common stock at an exercise price of $3.82 per share. The option is subject to a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. In May 2007, the compensation committee recommended, and our board of directors approved, the grant of 26,179 stock options to Mr. McEntire that include an accelerated vesting feature tied to the achievement of certain individual and corporate performance goals for 2007 and 2008. Mr. McEntire is entitled to certain benefits in connection with a termination of his employment upon a change in control discussed below under “—Potential Payments Upon Termination or Change in Control.”
Reyn E. Gallacher, Vice President, Finance, Chief Financial Officer and Assistant Secretary. Pursuant to a letter agreement dated January 3, 2006 between us and Mr. Gallacher, we agreed to employ Mr. Gallacher as our Controller beginning on January 6, 2006. Mr. Gallacher received an initial stock option grant of 10,472 shares upon joining Amedica. As of December 31, 2006, his base salary was $108,000. In January 2007, Mr. Gallacher began serving as our Vice President of Finance and his salary was increased to $130,000 effective January 1, 2007. Based upon our overall financial position; our product development progress; and his contributions towards implementing a new accounting system, managing the budget, forecasts and monthly financial reports, and implementing internal controls for the fiscal year ended December 31, 2006, and in connection with his promotion to our Vice President of Finance, Mr. Gallacher received an option to purchase 7,854 shares of our common stock at an exercise price of $3.82 per share. The option is subject to a four-year vesting schedule with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter.
Since March 2007, Mr. Gallacher has served as our Chief Financial Officer. In May 2007, in connection with his promotion, the compensation committee recommended, and our board of directors approved, an increase in Mr. Gallacher’s base salary to $150,000 per year retroactive to March 2007. Also in May 2007, the compensation committee recommended, and our board of directors approved, the grant of 10,472 stock options to Mr. Gallacher that include an accelerated vesting feature tied to the achievement of certain individual and corporate performance goals for 2007 and 2008. Mr. Gallacher is entitled to certain benefits in connection with a termination of his employment upon a change in control discussed below under “—Potential Payments Upon Termination or Change in Control.”
Eugene B. Jones, Former Vice President, Finance and Chief Financial Officer. Pursuant to a letter agreement dated April 2, 2004 between us and Mr. Jones, we agreed to employ Mr. Jones as our Vice President of Finance beginning April 19, 2004. Mr. Jones base salary for the fiscal year ended December 31, 2006 was $155,000. Mr. Jones resigned effective January 5, 2007 and received certain compensation in connection with his termination discussed under “—Potential Payments Upon Termination or Change in Control.”
Cameron G. Rouns, Former Vice President, Sales and Marketing. Pursuant to a letter agreement dated December 17, 2004 between us and Mr. Rouns, we agreed to employ Mr. Rouns as our Vice President of Sales and Marketing beginning January 4, 2005. Mr. Rouns base salary for the fiscal year ended December 31, 2006 was $136,000. Mr. Rouns resigned from this position effective December 1, 2006.
95
Our 2003 Stock Option Plan
All options granted to our employees, including our executive officers, under the 2003 Stock Option Plan are exercisable in accordance with the terms of an option agreement entered into at the time of the grant. Options are generally exercisable for a period of ten years, provided that if an employee is terminated without cause or leaves for any reason other than death or disability, the incentive stock options are generally exercisable within three months after termination of the employee’s employment to the extent then vested on the date of such termination. By contrast, non-qualified stock options are generally exercisable upon termination without cause for the full ten-year term, to the extent then vested on the date of the cessation of employment.
Outstanding Equity Awards At Fiscal Year-End
The following table shows grants of stock options outstanding on the last day of the fiscal year ended December 31, 2006 to each of the executive officers named in the Summary Compensation Table.
| Option Awards | |||||||||||
| Name |
Number
of Exercisable |
Number
of Unexercisable |
Option Exercise Price |
Option Expiration Date | |||||||
| Ashok C. Khandkar, Ph.D. |
209,425 | (1) | — | $ | 0.42 | 09/17/2013 | |||||
| Chief Executive Officer |
5,236 | 5,236 | (2) | $ | 2.29 | 12/15/2014 | |||||
| — | 10,472 | (3) | $ | 3.82 | 02/12/2016 | ||||||
| — | 10,472 | (4) | $ | 3.82 | 12/11/2016 | ||||||
| Warionex (“Jose”) Belen |
9,817 | — | (5) | $ | 2.29 | 06/15/2015 | |||||
| President |
— | 1,571 | (3) | $ | 3.82 | 02/12/2016 | |||||
| — | 7,854 | (4) | $ | 3.82 | 12/11/2016 | ||||||
| Bryan J. McEntire |
32,723 | 19,634 | (6) | $ | 0.96 | 06/08/2014 | |||||
| Vice President, Manufacturing and Research |
1,964 | 1,964 | (2) | $ | 2.29 | 12/15/2014 | |||||
| — | 7,854 | (3) | $ | 3.82 | 02/12/2016 | ||||||
| — | 7,854 | (4) | $ | 3.82 | 12/11/2016 | ||||||
| Reyn E. Gallacher |
— | 10,472 | (3) | $ | 3.82 | 02/12/2016 | |||||
| Vice President, Finance, Chief Financial Officer and |
— | 7,854 | (4) | $ | 3.82 | 12/11/2016 | |||||
| Assistant Secretary |
|||||||||||
| Eugene B. Jones |
13,090 | 19,634 | (6) | $ | 0.96 | 06/08/2014 | |||||
| Former Chief Financial Officer and Vice President, |
3,927 | 3,927 | (2) | $ | 2.29 | 12/15/2014 | |||||
| Finance |
— | 7,854 | (3) | $ | 3.82 | 02/12/2016 | |||||
| Cameron G. Rouns |
8,018 | 10,308 | (7) | $ | 2.29 | 03/20/2015 | |||||
| Former Vice President, Sales and Marketing |
— | 1,571 | (3) | $ | 3.82 | 02/12/2016 | |||||
| (1) |
The option vested as to 25% of the shares on September 17, 2003, the day this stock option was granted, and vested as to an additional 1/36th of the remaining unvested shares per month thereafter. |
| (2) |
The option vested as to 25% of the shares on December 15, 2005 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
| (3) |
The option vested as to 25% of the shares on February 12, 2007 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
| (4) |
The option vests as to 25% of the shares on December 11, 2007 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
| (5) |
The option vested as to 25% of the shares on June 15, 2006 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
96
| (6) |
The option vested as to 25% of the shares on June 8, 2005 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
| (7) |
The option vested as to 25% of the shares on March 20, 2006 and vests as to an additional 1/36th of the remaining unvested shares per month thereafter. |
Option Exercises And Stock Vested
The following table shows information regarding exercises of options to purchase our common stock held by each executive officer named in the Summary Compensation Table during the fiscal year ended December 31, 2006.
| Option Awards | |||||
| Name |
Number of Shares Acquired on Exercise |
Value Realized on Exercise | |||
| Ashok C. Khandkar, Ph. D. Chief Executive Officer |
— | $ | — | ||
| Warionex (“Jose”) Belen President |
— | — | |||
| Bryan J. McEntire Vice President, Manufacturing and Research |
— | — | |||
| Reyn E. Gallacher Vice President, Finance, Chief Financial Officer and Assistant Secretary |
— | — | |||
| Eugene B. Jones Former Vice President, Finance and Chief Financial Officer |
2,199 | 6,300 | |||
| Cameron G. Rouns Former Vice President, Sales and Marketing |
— | — | |||
| (1) | Amounts shown in this column do not necessarily represent actual value realized from the sale of the share acquired upon exercise of options because in many cases the shares are not sold on exercise but continue to be held by the executive officer exercising the option. The amounts shown represent the difference between the option exercise price and the fair market value on the date of exercise, which is the estimated amount that would have been realized if the shares had been sold immediately upon exercise. |
Pension Benefits
We do not have any qualified or non-qualified defined benefit plans.
Nonqualified Deferred Compensation
We do not have any nonqualified defined contribution plans or other deferred compensation plans.
Potential Payments Upon Termination or Change in Control
We have entered into certain agreements and maintain certain plans that may require us to make certain payments and/or provide certain benefits to the executive officers named in the Summary Compensation Table in the event of a termination of employment or change in control.
Termination of Employment and Change in Control Arrangements
Ashok C. Khandkar, Ph.D., Chief Executive Officer. Pursuant to our severance agreement with Dr. Khandkar, dated May 23, 2005, within one year following a change in control, in the event that Dr. Khandkar’s employment is terminated by us other than for cause (but not including termination due to death
97
or disability) or he resigns for good reason, we are required to pay him, in addition to any payments due for services rendered prior to his termination, a lump sum payment of an amount equal to three times his highest annual base salary and bonus payments during the preceding three-year period, including the year of his termination. In addition, all of his outstanding options shall become fully vested. If Dr. Khandkar had been terminated under the above referenced circumstances on December 31, 2006, he would have been entitled to receive $571,620 as a severance payment and all outstanding options would be fully vested, the fair value of which would have been $14,000.
Warionex (“Jose”) Belen, President. Pursuant to our severance agreement with Mr. Belen, dated February 14, 2006, within one year following a change in control, in the event that Mr. Belen’s employment is terminated by us other than for cause (but not including termination due to death or disability) or he resigns for good reason, we are required to pay him, in addition to any payments due for services rendered prior to his termination, a lump sum payment of an amount equal to two times his highest annual base salary and bonus payments during the preceding three-year period, including the year of his termination. In addition, all of his outstanding options shall become fully vested. If Mr. Belen had been terminated under the above referenced circumstances on December 31, 2006, he would have been entitled to receive $311,400 as a severance payment and all outstanding options would be fully vested, the fair value of which would have been $30,910.
Bryan J. McEntire, Vice President, Manufacturing and Research. Pursuant to our severance agreement with Mr. McEntire, dated May 23, 2005, within one year following a change in control, in the event that Mr. McEntire’s employment is terminated by us other than for cause (but not including termination due to death or disability) or he resigns for good reason, we are required to pay him, in addition to any payments due for services rendered prior to his termination, a lump sum payment of an amount equal to two times his highest annual base salary and bonus payments during the preceding three-year period, including the year of his termination. In addition, all of his outstanding options shall become fully vested. If Mr. McEntire had been terminated under the above referenced circumstances on December 31, 2006, he would have been entitled to receive $312,100 as a severance payment and all outstanding options would be fully vested, the fair value of which would have been $67,800.
Reyn E. Gallacher, Vice President, Finance, Chief Financial Officer and Assistant Secretary. Pursuant to our severance agreement with Mr. Gallacher, dated March 27, 2007, within one year following a change in control, in the event that Mr. Gallacher’s employment is terminated by us other than for cause (but not including termination due to death or disability) or he resigns for good reason, we are required to pay him, in addition to any payments due for services rendered prior to his termination, a lump sum payment of an amount equal to two times his highest annual base salary and bonus payments during the preceding three-year period, including the year of his termination. In addition, all of his outstanding options shall become fully vested. If Mr. Gallacher had been terminated under the above referenced circumstances on December 31, 2006, he would have been entitled to receive $216,000 as a severance payment and all outstanding options would be fully vested, the value of which would have been $4,200.
If it is determined that the amounts payable to each executive officer under his severance agreement, when considered together with any other amounts payable to the executive officer in connection with a change in control of Amedica, cause these payments to be treated as excess parachute payments under Section 280G of the Internal Revenue Code, then we will be required to make an additional “gross up” payment in order to pay for any additional tax imposed on him pursuant to Section 4999 of the Internal Revenue Code. If Dr. Khandkar had been terminated on December 31, 2006 as a result of a change in control, he would have been entitled to receive approximately $238,135 as an additional “gross up” payment.
As defined in the severance agreements with our executive officers:
| • | “Cause” means (i) the executive’s commission of a felony (other than through vicarious liability or through a motor vehicle offense); (ii) the executive’s material disloyalty or dishonesty to us; (iii) an act of fraud, embezzlement or misappropriation of funds by the executive; (iv) a material breach by the executive of any material provision of the severance agreement or any other agreement with us, which |
98
| breach is not cured within 30 days after notice to the executive by us of the breach; or (v) the executive’s refusal to carry out a lawful written directive from our board of directors. Any determination of “cause” will be made by a majority of the members of our board voting on such determination. |
| • | “Good Reason” means without the executive’s consent: (i) a material change in the principal location at which the executive performs his duties for us to a new location that is at least 50 miles from the prior location; or (ii) a material change in the executive’s authority, functions, duties or responsibilities, which would cause his position with us to become of less responsibility, importance or scope than his position on the date of entering in the severance agreement or as of any subsequent date prior to a change in control, provided, however, that this material change is not in connection with the termination of the executive’s employment by us for any reason. |
| • |
“Change in Control” means: (i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, becomes the “beneficial owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of our securities representing 50% or more of the total voting power represented by our then outstanding voting securities pursuant to a transaction or a series of related transactions of which our board does not approve; (ii) a merger or consolidation of us, whether or not approved by our board, other than a merger or consolidation which would result in our voting securities outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or the parent of such corporation) at least 50% of the total voting power represented by our voting securities or the surviving entity or parent of the corporation outstanding immediately after the merger or consolidation; or (iii) our stockholders approve an agreement for the sale or disposition by us of all or substantially all of our assets. |
Eugene B. Jones, Former Vice President, Finance and Chief Financial Officer. Pursuant to an agreement we made with Mr. Jones dated January 5, 2007, regarding his separation from us, we paid Mr. Jones a lump sum of $95,566. In addition, we granted Mr. Jones a non-qualified stock option exercisable for up to 17,016 shares of our common stock, which expires on June 30, 2014. All of the stock options previously granted to Mr. Jones were terminated upon his separation from us.
Change in Control Arrangements Under Our 2003 Stock Option Plan
All options granted under our 2003 Stock Option Plan become fully vested upon a change in control provided that the employee, officer, director, surgeon advisors or other consultant holds the position on the date of such change in control. If we are to be consolidated with or acquired by another entity in a merger or sale of all or substantially all of our assets, our board of directors or the board of directors of any entity assuming our obligations under the plan, will, as to outstanding options, take any one or more of the following actions pursuant to our 2003 Stock Option Plan:
| • | make appropriate provision for the continuation of options granted under the plan by substituting on an equitable basis for the shares of our common stock then subject to these options either the consideration payable with respect to those outstanding shares of our common stock in connection with the merger or sale of our assets or securities of any successor or acquiring entity; |
| • | upon written notice to a participant, provide that the participant’s options must be exercised within a specified number of days of the date of that notice, to the extent then exercisable or, at the discretion of our board of directors, or upon a change in control, all options being made fully exercisable, at the end of which period the options will terminated; or |
| • | terminate all options in exchange for a cash payment equal to the excess of the fair value, as defined in the plan, of the shares of our common stock subject to these options over the exercise price thereof, to the extent then exercisable or, at the discretion of our board of directors, or upon a change in control, all options being made fully exercisable. |
99
Director Compensation
The following table shows the total compensation paid or accrued during the fiscal year ended December 31, 2006 to each of our non-employee directors.
| Name |
Option Awards (1) | Total | ||||
| Max Link, Ph.D.(2) |
$ | 4,804 | $ | 4,804 | ||
| Bradford S. Goodwin(3) |
— | — | ||||
| Lawrence D. Dorr, M.D.(4) |
169 | 169 | ||||
| Aaron A. Hofmann, M.D.(5) |
1,560 | 1,560 | ||||
| Gregg R. Honigblum(6) |
— | — | ||||
| Peter D. Meldrum(7) |
3,911 | 3,911 | ||||
| Rohit Patel(8) |
2,030 | 2,030 | ||||
| (1) | The dollar amounts in this column represent the compensation cost for the year ended December 31, 2006 of stock option awards granted in and prior to 2006. These amounts have seen calculated in accordance with FASB Statement No. 123 (revised), “Share-Based Payment,” or SFAS No. 123R, using the Black Scholes Valuation model. See Notes 1 and 7 to our financial statements included elsewhere in this prospectus for details as to assumptions used to determine the fair value of the option awards. See also our discussion of stock-based compensation under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates—Stock-Based Compensation.” |
| (2) | As of December 31, 2006, Dr. Link held options to purchase 65,390 shares of common stock, of which 56,555 were vested. |
| (3) | Mr. Goodwin joined our board of directors on May 6, 2007. |
| (4) | As of December 31, 2006, Dr. Dorr held options to purchase 3,927 shares of common stock, of which none were vested. |
| (5) | As of December 31, 2006, Dr. Hofmann held options to purchase 11,780 shares of common stock, of which 2,945 were vested. |
| (6) | Although, Mr. Honigblum received no compensation for his services as a director in fiscal year 2006, he is affiliated with an entity that received compensation from us in connection with our offerings of shares of our preferred stock. For a detailed description of these transactions, see “Certain Relationships and Related Person Transactions.” |
| (7) | Mr. Meldrum resigned from our board of directors effective December 11, 2006. As of December 11, 2006, Mr. Meldrum held options to purchase 158,950 shares of common stock, all of which were vested. |
| (8) | As of December 31, 2006, Mr. Patel held options to purchase 43,194 shares of common stock, of which 34,359 were vested. |
Director Compensation Policy
Each of our non-employee directors was granted 3,927 stock options pursuant to our 2003 Stock Option Plan as remuneration for his service on our board of directors for the year ended December 31, 2006. Beginning in 2007, newly appointed non-employee directors will now receive an initial grant of 10,472 stock options in addition to the 3,927 stock options that we issue on an annual basis. Because of this change, our board of directors approved a grant of an additional 6,545 stock options to Dr. Dorr and a grant of 10,472 stock options to Mr. Honigblum in May 2007 so that each of these directors will have been granted a total of 10,472 stock options as a result of their recent appointments to our board of directors. Generally, these stock options vest over a four-year period, with 25% of the shares vesting on the first anniversary of the date of grant, and 1/36th of the remaining unvested shares vesting monthly thereafter. On June 18, 2007, our board of directors authorized us to amend stock options previously granted to our directors to allow our directors to purchase all unvested shares under their options, subject to our right to repurchase the shares so acquired before they would otherwise vest in accordance with our normal four year vesting schedule. Only one of our directors, Bradford S. Goodwin, has
100
elected to have his only stock option amended to allow for his purchase of unvested shares subject to our repurchase right. All stock options granted to our directors become fully vested immediately upon the occurrence of a change in control or a sale of all or substantially all of our assets. In the case of the amended stock option granted to Mr. Goodwin, all of the shares that he may acquire by exercise of his option shall no longer be subject to repurchase immediately upon the occurrence of a change in control or a sale of all or substantially all of our assets. We also reimburse our directors for reasonable out-of-pocket expenses incurred by them in connection with the attendance at quarterly board meetings.
On June 18, 2007 our board of directors adopted a new director compensation policy, for fiscal year 2007, that provides for, in addition to the grant of stock options, cash payments to our non-employee directors for their service as members of the board of directors and for service as members and chairs of board committees. Under the new policy, we will pay each of our non-employee directors a $20,000 annual retainer to be paid quarterly, $1,500 for in person attendance at each board meeting, and $1,000 for participation at each board meeting conducted by telephone conference. We will pay a $10,000 annual retainer for service as Chair of our audit committee, and a $7,500 annual retainer for service as Chair of each of our compensation committee and nominating and governance committee. Other non-employee members of each of our board committees will be paid a $3,750 annual retainer for their service as member of the committee. We also will pay each non-employee member of a board committee $1,500 for in person attendance at each committee meeting, and $1,000 for participation at each committee meeting conducted by telephone conference, provided that such meetings are not held in conjunction with an in person meeting of the board. None of these amounts earned in fiscal 2007 will be paid until after completion of this offering. Thereafter, payments will be made on a quarterly basis.
Employee Benefit Plans
2003 Stock Option Plan
Our 2003 Stock Option Plan was approved by our board of directors and our stockholders on August 3, 2003. The board of directors believes the availability of stock options is an important factor in our ability to attract or retain qualified employees and to provide incentives for them to exert their best interests on our behalf.
All of our employees, officers, directors, surgeon advisors and other consultants are eligible to participate in the plan. The 2003 Stock Option Plan is administered by our board of directors, which designates the price and other terms and conditions of any award granted under the plan. Subject to the provisions of the plan, our board of directors may adopt and amend rules and regulations relating to the administration of the plan. Our board of directors may also delegate authority for administration of the plan to a committee of the board of directors.
As of March 31, 2007, we had reserved a total of 1,047,121 shares of our common stock for issuance under the 2003 Stock Option Plan. In May 2007, we reserved an additional 130,890 shares of our common stock for issuance under the plan. As of March 31, 2007, options for 905,198 shares are outstanding under the plan. Options to purchase 48,486 shares of common stock have been exercised as of March 31, 2007. The 2003 Stock Option Plan will terminate on August 7, 2013. The plan may be terminated at an earlier date by vote of the shareholders or our board of directors; provided, however, that any such earlier termination shall not affect any options issued prior to the effective date of such termination.
The 2003 Stock Option Plan permits the grant of both incentive stock options, or ISOs, and non-statutory stock options, or NSOs. Shares of common stock awarded under the plan may be authorized and unissued shares of common stock or shares of common stock held by us in our treasury. If any option granted under the plan expires, terminates or is cancelled, the shares of common stock again become available for issuance under the plan.
Our board of directors determines the persons to whom options are granted, the option price, the number of shares of common stock to be covered by each option, the period of each option, the times at which options may be exercised, and whether the option is an ISO, as defined in the Internal Revenue Code, or an NSO.
101
All options granted under the plan are exercisable in accordance with the terms of an option agreement entered into at the time of the grant. Options are generally exercisable for a period of ten years, provided that if an employee is terminated or leaves without cause, the ISOs are generally exercisable within three months after termination of the employee’s employment to the extent then vested on the date of such termination. By contrast, NSOs are generally exercisable upon termination without cause for the full ten-year term, to the extent then vested on the date of such cessation. All options become fully vested upon a change of control provided that the employee, officer, director, surgeon advisors or other consultant holds such position on the date of such change of control.
If we are to be consolidated with or acquired by another entity in a merger or sale of all or substantially all of our assets, our board of directors or the board of directors of any entity assuming our obligations under the plan, will, as to outstanding options, take any one or more of the following actions pursuant to our 2003 Stock Option Plan:
| • | make appropriate provision for the continuation of such options by substituting on an equitable basis for the shares of our common stock then subject to such options either the consideration payable with respect to such outstanding shares of our common stock in connection with the merger or sale of our assets or securities of any successor or acquiring entity; |
| • | upon written notice to a participant, provide that the participant’s options must be exercised within a specified number of days of the date of such notice, to the extent then exercisable or, at the discretion of our board of directors, or upon a change of control, all options being made fully exercisable, at the end of which period the options will terminated; or |
| • | terminate all options in exchange for a cash payment equal to the excess of the fair market value, as defined in the plan, of the shares of our common stock subject to such options over the exercise price thereof, to the extent then exercisable or, at the discretion of our board of directors, or upon a change of control, all options being made fully exercisable. |
After the effective date of the 2007 Stock Plan described below, we will grant no further stock options under the 2003 Stock Option Plan.
2007 Employee, Director and Consultant Stock Plan
We anticipate that our board of directors will adopt a 2007 Employee, Director and Consultant Stock Plan, or the 2007 Stock Plan. Our 2007 Stock Plan will become effective on the date that the registration statement is declared effective. This plan provides for the grant of incentive stock options, nonqualified stock options, restricted and unrestricted stock awards and other stock-based awards. Our officers, employees, consultants, advisors and directors will be eligible to receive awards under this plan, however, incentive stock options may only be granted to our employees. Upon effectiveness, 2,000,000 shares of common stock will be reserved for issuance under this plan. In addition, the 2007 Stock Plan contains an “evergreen” provision, which allows for an annual increase in the number of shares available for issuance under the plan on the first day of each fiscal year during the period beginning on the first day of fiscal year 2008, and ending on the second day of fiscal year 2017. The annual increase in the number of shares shall be equal to the lowest of:
| • | 750,000 shares; |
| • | 5% of the number of shares of our common stock outstanding on the first day of the applicable fiscal year; and |
| • | an amount determined by our board of directors. |
Upon completion of this offering, it is anticipated that this plan shall be administered by our compensation committee. The compensation committee will determine the terms of options and other awards granted pursuant to this plan, including:
| • | the determination of which employees, directors and consultants will be granted options and other awards; |
102
| • | the number of shares subject to options and other awards; |
| • | the exercise price of each option which may not be less than fair market value on the date of grant; |
| • | the schedule upon which options become exercisable; |
| • | the purchase price, if any, and number of shares subject to each award; |
| • | the termination or cancellation of provisions applicable to options; the terms and conditions of other awards, including conditions for repurchase, termination or cancellation, issue price and repurchase price; and |
| • | all other terms and conditions upon which each award may be granted in accordance with the 2007 Stock Plan. |
No participant may receive awards for over 300,000 shares of common stock in any fiscal year.
In addition, our compensation committee may, with the consent of the affected plan participants, reprice or otherwise amend outstanding awards consistent with the terms of the 2007 Stock Plan.
The maximum term of options granted under the 2007 Stock Plan is ten years and our plan terminates in July 2017.
Upon a merger or other reorganization event, our board of directors, may, in their sole discretion, take any one or more of the following actions pursuant to our 2007 Stock Plan:
| • | provide that all options shall be assumed or substituted by the successor corporation; |
| • | upon written notice to a participant, provide that the participant’s unexercised options will terminate immediately prior to the consummation of such transaction unless exercised by the participant; |
| • | in the event of a merger pursuant to which holders of our common stock will receive a cash payment for each share surrendered in the merger, make or provide for a cash payment to the participants equal to the difference between the merger price times the number of shares of our common stock subject to such outstanding options (at prices not in excess of the merger price), and the aggregate exercise price of all such outstanding options, in exchange for the termination of such options; and |
| • | provide that outstanding awards shall be assumed or substituted by the successor corporation, become realizable or deliverable, or restrictions applicable to an award will lapse, in whole or in part, prior to or upon the merger or reorganization event. |
In addition, under our 2007 Stock Plan, all options become fully vested upon a change of control provided that the employee, officer, director, surgeon consultants or other consultant holds such position on the date of such change of control. Our 2007 Stock Plan provides similar change in control vesting provisions for restricted stock granted under the 2007 Stock Plan and allows the Board to make appropriate adjustments for other stock-based awards.
401(k) Plan
Our employee savings plan is a tax-qualified profit sharing plan that includes a “cash-or-deferred” (or 401(k)) feature. The plan is intended to satisfy the requirements of Section 401 of the Internal Revenue Code. Our employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and have a like amount contributed to the plan. In addition, we may make discretionary and/or matching contributions to the plan in amounts determined annually by our board of directors. Effective upon the completion of our first full payroll period following July 1, 2007, we plan to begin matching the contributions of our employees who participate in our 401(k) plan as follows: a match of 100% on the first 3% of compensation contributed by a plan participant and a match of 50% on amounts above 3%, up to 5%, of compensation contributed by a plan participant.
103
Limitation of Directors’ Liability and Indemnification
The Delaware General Corporation Law authorizes corporations to limit or eliminate, subject to certain conditions, the personal liability of directors to corporations and their stockholders for monetary damages for breach of their fiduciary duties. Our amended and restated certificate of incorporation limits the liability of our directors to the fullest extent permitted by Delaware law.
We have obtained director and officer liability insurance to cover liabilities our directors and officers may occur in connection with their services to us, including matters arising under the Securities Act. Our amended and restated certificate of incorporation and amended and restated bylaws also provide that we will indemnify any of our directors and officers who, by reason of the fact that he or she is one of our officers or directors, is involved in a legal proceeding of any nature. We will repay certain expenses incurred by a director or officer in connection with any civil or criminal action or proceeding, specifically including actions by us or in our name (derivative suits). These indemnifiable expenses include, to the maximum extent permitted by law, attorney’s fees, judgments, civil or criminal fines, settlement amounts and other expenses customarily incurred in connection with legal proceedings. A director or officer will not receive indemnification if he or she is found not to have acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interest.
Prior to the completion of this offering, we plan to enter into agreements to indemnify our directors and officers. These agreements, among other things, will indemnify and advance expenses to our directors and officers for certain expenses, including attorney’s fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by us arising out of such person’s services as our director or officer, or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and officers.
This limitation of liability and the indemnification of our directors and officers does not affect the availability of equitable remedies. In addition, we have been advised that in the opinion of the SEC, indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act and is therefore unenforceable.
There is no pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for indemnification under the agreements described in this section.
104
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Since January 1, 2004, with the approval of our board of directors, we have engaged in the transactions described below with our directors, executive officers and beneficial owners of more than 5% of our common stock, on an as-converted basis, and affiliates of our directors, executive officers and 5% stockholders. In addition, we effected a 1-for-3.82 reverse stock split of our common stock on July 26, 2007.
Royalty Payments
One of our co-founders and a member of our board of directors, Aaron A. Hofmann, M.D., is also the sole member and president of Joint Enterprises, L.C., a Utah limited liability company. We and Joint Enterprises, L.C. previously entered into an Assignment Agreement, dated August 1, 2001, which has been amended as of August 12, 2005. Pursuant to this agreement, we acquired rights to the PreVent Cement Restrictor in exchange for our agreement to pay a one-time payment of $25,000 and to pay royalties equal to $2.50 per unit. Joint Enterprises, L.C. also has the option to elect to receive nonqualified stock options to purchase shares of our common stock in lieu of cash payments, subject to approval by our board of directors. We made the $25,000 payment to Dr. Hofmann in September 2004. As of the date hereof, no units of this product have been sold and no royalties for this product have been paid pursuant to this agreement.
Issuance of Stock and Warrants
We have completed various offerings of shares of our Series A, Series B, Series C and Series D convertible preferred stock through Creation Capital LLC, our placement agent for each of these offerings. Gregg R. Honigblum is the Chief Executive Officer and a 50% co-owner of Creation Capital LLC and he joined our board of directors in December 2006, subsequent to our Series A, Series B and Series C convertible preferred stock offerings but prior to our Series D convertible preferred stock offering. In connection with our third closing of our Series A convertible preferred stock offering, which occurred on January 28, 2004, we raised $2.3 million in gross proceeds and sold 3,805,018 shares at $0.60 per share, and we paid our placement agent $182,640 as commission and $20,875 for expenses in connection with the third closing of the offering, of which Mr. Honigblum received $48,125 from Creation Capital LLC as a result of his ownership interest therein. In connection with our Series B convertible preferred stock offering, closings for which occurred on October 25, 2004 and November 9, 2004, we raised an aggregate of $6.0 million in gross proceeds and sold a total of 5,000,000 shares at $1.20 per share, and we paid our placement agent $480,000 as commission and $40,000 for expenses in connection with the closings, of which Mr. Honigblum received $135,000 from Creation Capital LLC as a result of his ownership interest therein. In connection with our Series C convertible preferred stock offering, which occurred on February 24, 2006, we raised $16.8 million in gross proceeds and sold 8,400,000 shares at $2.00 per share, and we paid our placement agent $1,511,265 as commission and $75,000 for expenses in connection with the offering, of which Mr. Honigblum received $759,500 from Creation Capital LLC as a result of his ownership interest therein. In connection with our Series D convertible preferred stock offering, closings for which occurred on April 17, 2007 and April 27, 2007, we raised $13.4 million in gross proceeds and sold 4,456,500 shares at $3.00 per share, and we paid our placement agent $758,870 as commission and $100,000 for expenses in connection with the closings, of which Mr. Honigblum received $300,000 from Creation Capital LLC as a result of his ownership interest therein. In addition, in connection with our Series C convertible preferred stock offering, we agreed to pay Creation Capital LLC a transaction fee in the event that prior to a registered offering of our securities, we complete a stock sale, merger, tender offer, recapitalization or asset sale by February 24, 2009 that results in a change in control of us. The amount of the transaction fee payable to Creation Capital LLC upon such an event would be 1.5%-3% of the aggregate consideration payable to us in connection with the transaction, up to $2,500,000.
In addition to the cash compensation we paid to Creation Capital LLC for the services it provided as the placement agent for our convertible preferred stock offerings, we agreed to issue warrants to purchase shares of our preferred stock to Creation Capital LLC. In connection with these offerings, we issued to Creation Capital
105
LLC, or at its request to certain of its designees, including one of our directors and a principal stockholder, warrants to purchase 2,100,000 shares of Series A convertible preferred stock, 500,000 shares of Series B convertible preferred stock, 1,203,750 shares of Series C convertible preferred stock and 253,290 shares of Series D convertible preferred stock, at an exercise price equal to 110% of the offering price of the underlying convertible preferred stock, or $0.66 per share, $1.32 per share, $2.20 per share and $3.30 per share of our Series A, Series B, Series C and Series D convertible preferred stock, respectively. The warrants to purchase shares of our Series A, Series B and Series C convertible preferred stock are currently exercisable through the seventh anniversary of the date of issuance of such warrants, or January 28, 2011, November 9, 2011 and February 24, 2013, respectively, and upon completion of the offering, the warrants to purchase shares of our Series D convertible preferred stock will become exercisable through the seventh anniversary of the date of issuance of such warrants, or April 27, 2014.
The following table summarizes the purchases of shares of our Series A (third closing), Series B, Series C and Series D convertible preferred stock by, as well as the issuance of warrants to purchase shares of our convertible stock to, our directors and beneficial owners of 5% or more of our common stock, on an as-converted basis. None of our executive officers participated in any of the offerings.
| Name |
Series A Preferred |
Series A Preferred Warrants |
Series B Preferred |
Series B Preferred |
Series C Preferred |
Series C Preferred Warrants |
Series D Preferred |
Series D Preferred Warrants |
||||||||||||||||
| Directors |
||||||||||||||||||||||||
| Max Link, Ph.D. |
— | — |
|
— | — | 100,000 | — | 35,000 | — | |||||||||||||||
| Aaron A. Hofmann, M.D. |
— |
|
— | — | — | 250,000 | — | 35,000 | (1) | — | ||||||||||||||
| Lawrence D. Dorr, M.D. |
— |
|
— |
|
— | — | — | — | 130,000 | (2) | — | |||||||||||||
| Gregg R. Honigblum |
62,501 | (3) | 716,673 | (4) | — | 165,875 | (5) | 12,500 | (6) | 452,125 | (7) | — | 111,645 | (8) | ||||||||||
| Rohit Patel |
— |
|
— |
|
15,000 | (9) | — | 25,000 | (10) | — | 35,000 | (11) | — | |||||||||||
| Principal stockholder |
||||||||||||||||||||||||
| Vestal Venture Capital |
— |
|
130,469 | (12) | 805,500 | (13) | 65,751 | (14) | 1,112,500 | — | 400,000 | — | ||||||||||||
| (1) | Consists of 35,000 shares of our Series D convertible preferred stock purchased by Dr. Hofmann’s spouse. |
| (2) | Consists of 130,000 shares of our Series D convertible preferred stock purchased by Dr. Dorr and his spouse. |
| (3) | Mr. Honigblum is the Chief Executive Officer and a 50% co-owner of Creation Capital LLC and he is currently a member of our board of directors. In accordance with the rules of the SEC, Mr. Honigblum is deemed to beneficially own 50% of the 125,001 shares of our Series A convertible preferred stock purchased by Creation Capital LLC in connection with the third closing of our offering of shares of our Series A convertible preferred stock. Mr. Honigblum disclaims beneficial ownership of the shares held by Creation Capital LLC except to the extent of his proportionate pecuniary interest therein. |
| (4) | See footnote (3). The warrants noted above consist of warrants to acquire 716,673 shares of our Series A convertible preferred stock issued to Mr. Honigblum at the request of Creation Capital, which Creation Capital LLC was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of the first, second and third closings of our offering of shares of our Series A convertible preferred stock. The warrants noted above exclude warrants to acquire 822,506 shares of Series A convertible preferred stock held by Michael Morris, the President and the other 50% co-owner of Creation Capital LLC. |
| (5) | See footnote (3). The warrants noted above consist of warrants to acquire 165,875 shares of our Series B convertible preferred stock issued to Mr. Honigblum at the request of Creation Capital LLC, which Creation Capital LLC was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of our offering of shares of our Series B convertible preferred stock. The warrants noted above exclude warrants to acquire an additional 250,000 shares of our Series B convertible preferred stock, which we were obligated to issue to Creation Capital LLC as partial compensation for such services. The warrants noted above exclude warrants to acquire 175,874 shares of Series B convertible preferred stock held by Michael Morris. |
| (6) | See footnote (3). Mr. Honigblum is deemed to beneficially own 50% of the 25,000 shares of our Series C convertible preferred stock purchased by Creation Capital LLC in connection with our offering of shares of |
106
| our Series C convertible preferred stock. Mr. Honigblum disclaims beneficial ownership of the shares held by Creation Capital LLC except to the extent of his proportionate pecuniary interest therein. |
| (7) | See footnote (3). The warrants noted above consist of warrants to acquire 452,125 shares of our Series C convertible preferred stock issued to Mr. Honigblum at the request of Creation Capital LLC, which Creation was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of our offering of our Series C convertible preferred stock. The warrants noted above exclude warrants to acquire 424,125 shares of Series C convertible preferred stock held by Michael Morris. |
| (8) | See footnote (3). Mr. Honigblum is deemed to beneficially own 50% of the warrants to acquire 223,290 shares of our Series D convertible preferred stock, which Creation Capital LLC received as partial compensation for the services it provided as our placement agent in connection with the completion of our offering of shares of our Series D convertible preferred stock. Mr. Honigblum disclaims beneficial ownership of the warrants held by Creation Capital LLC except to the extent of his proportionate pecuniary interest therein. |
| (9) | Consists of 15,000 shares of our Series B convertible preferred stock previously purchased by Mr. Patel, which have since been gifted to his daughter, granddaughter and two unaffiliated parties. |
| (10) | Consists of 25,000 shares of our Series C convertible preferred stock purchased by Mr. Patel, which have since been gifted to The Patel Family Trust U/A/D November 7, 1996, of which Mr. Patel and his spouse are the sole beneficiaries. |
| (11) | Consists of 35,000 shares of our Series D convertible preferred stock purchased by The Patel Family Trust U/A/D November 7, 1996, of which Mr. Patel and his spouse are the sole beneficiaries. |
| (12) | Consists of warrants to acquire 68,694 and 127,344 shares of our Series A convertible preferred stock issued to Lyonshare Venture Capital and Vestal Venture Capital, respectively, at the request of Creation Capital LLC, which Creation Capital LLC was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of an offering of our Series A convertible preferred stock. Allan R. Lyons is the managing member and sole owner of 21st Century Strategic Investment Planning, L.C., the general partner for both Vestal Venture Capital and Lyonshare Venture Capital. Mr. Lyons disclaims beneficial ownership of the shares held by Vestal Venture Capital and Lyonshare Venture Capital except to the extent of his proportionate pecuniary interest therein. |
| (13) | Consists of 130,000 and 675,500 shares of our Series B convertible preferred stock purchased by Lyonshare Venture Capital and Vestal Venture Capital, respectively. See footnote (12). |
| (14) | Consists of warrants to acquire 16,013 and 29,738 shares of our Series B convertible preferred stock, issued to Lyonshare Venture Capital and Vestal Venture Capital, respectively, at the request of Creation Capital LLC, which Creation Capital LLC was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of our offering of our Series B convertible preferred stock. Also includes warrants held by Allan R. Lyons to acquire up to 20,000 shares of our Series B convertible preferred stock. See footnote (12). |
Registration Rights
The holders of 1,457,830 shares of common stock, assuming the conversion of our convertible preferred stock, and holders of 1,062,022 shares of common stock, assuming the exercise of preferred stock warrants and further assuming the conversion of such shares of convertible preferred stock, have entered into an agreement with us that provides certain registration rights to these holders and certain future transferees of their securities. Such holders include the above-listed directors and holders of 5% or more of our common stock, on an as-converted basis. See “Description of Capital Stock—Registration Rights” on page 111 for a description of these rights.
Stock Option Grants
We have granted options to purchase shares of our common stock to our executive officers and directors. See “Executive Compensation—Summary Compensation Table” on page 92, “Executive Compensation—Grants of Plan-Based Awards” on page 93, “Executive Compensation—Outstanding Equity Awards at Fiscal Year-End” on page 96 and “Executive Compensation—Director Compensation” on page 100.
107
Change in Control Agreements
We have entered into severance agreements with our executive officers as described in the section of this prospectus entitled “Executive Compensation—Potential Payments Upon Termination or Change in Control” on page 97.
Policy for Approval of Related Person Transactions
We believe that all the transactions described above were made on terms no less favorable to us than those that could have been obtained from unaffiliated third parties. With the exception of transactions in which related parties participated on the same terms as those of other participants who were not related parties, our board of directors reviewed and approved the transactions with each related party, namely our directors, executive officers and beneficial owners of more than 5% of our common stock, on an as-converted basis, and affiliates of our directors, executive officers and 5% stockholders, and reviewed the material facts as to a related party’s relationship or interest in a transaction that were disclosed to our board of directors prior to our board of directors’ consideration of a transaction with a related party. The transactions involving related parties were approved by our board of directors, including all of our directors who were not interested in these transactions.
Following this offering, all future related party transactions will be approved by our audit committee. Pursuant to the written charter of our audit committee, the audit committee is responsible for reviewing and approving, prior to our entry into any transaction involving related parties, all transactions in which we are a participant and in which any parties related to us has or will have a direct or indirect material interest.
In reviewing and approving these transactions, the audit committee shall obtain, or shall direct our management to obtain on its behalf, all information that the committee believes to be relevant and important to a review of the transaction prior to its approval. Following receipt of the necessary information, a discussion shall be held of the relevant factors, if deemed to be necessary by the committee, prior to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of the committee. No related party transaction shall be entered into prior to the completion of these procedures.
The audit committee or its chairman, as the case may be, shall approve only those related party transactions that are determined to be in, or not inconsistent with, the best interests of us and our stockholders, taking into account all available facts and circumstances as the committee or the chairman determines in good faith to be necessary. No member of the audit committee shall participate in any review, consideration or approval of any related party transaction with respect to which the member or any of his or her immediate family members is the related party.
108
The following table sets forth certain information regarding the beneficial ownership of our common stock as of June 30, 2007, on an as-converted basis, and as adjusted to reflect the sale of our common stock offered by this prospectus by:
| • | the executive officers named in the summary compensation table; |
| • | each of our directors; |
| • | all of our current directors and executive officers as a group; and |
| • | each stockholder known by us to own beneficially more than 5% of our common stock. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of June 30, 2007, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of ownership is based on 10,668,818 shares of common stock outstanding on June 30, 2007, which assumes the conversion of all outstanding shares of preferred stock into common stock, and 15,318,818 shares of common stock outstanding after the completion of this offering.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. The address for each director and executive officer listed is: c/o Amedica Corporation, 615 Arapeen Drive, Suite 302, Salt Lake City, Utah 84108.
| Name and Address of Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
||||||
| Before Offering(1) |
After Offering |
|||||||
| Directors and Named Executive Officers: |
||||||||
| Ashok C. Khandkar, Ph.D.(2) |
1,267,450 |
11.6 | % | 8.2 | % | |||
| Aaron A. Hofmann, M.D.(3) |
1,255,243 |
11.8 | % | 8.2 | % | |||
| Gregg R. Honigblum(4) |
398,250 |
3.6 |
% |
2.5 | % | |||
| Max Link, Ph.D.(5) |
205,386 |
1.9 | % | 1.3 | % | |||
| Rohit Patel(6) |
51,372 |
* | * | |||||
| Bryan J. McEntire(7) |
53,554 |
* | * | |||||
| Eugene B. Jones(8) |
36,648 |
* | * | |||||
| Lawrence D. Dorr, M.D.(9) |
34,031 | * | * | |||||
| Warionex (“Jose”) Belen(10) |
21,312 |
* | * | |||||
| Bradford S. Goodwin(11) |
10,471 |
* | * | |||||
| Reyn E. Gallacher(12) |
9,161 |
* | * | |||||
| Cameron G. Rouns |
— | — | — | |||||
| All directors and executive officers as a group (13 individuals)(13) |
3,319,180 |
29.0 |
% |
20.6 |
% | |||
| Five Percent Stockholder: |
||||||||
| Vestal Venture Capital(14) 92 Hawley Street, P. O. Box 1330 Binghamton, New York 13902 |
977,258 | 9.1 |
% |
6.4 | % | |||
| * | Represents beneficial ownership of less than 1% of the shares of our common stock. |
| (1) | Based on 10,668,818 shares of common stock outstanding on June 30, 2007, which assumes the conversion of all outstanding shares of preferred stock into common stock. Unless otherwise indicated, each person or entity listed has sole investment and voting power with respect to the shares listed. |
109
| (2) | Consists of 523,560 shares of our common stock and options to acquire a total of 220,330 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007 held by Dr. Khandkar and 523,560 shares of our common stock held by Dr. Khandkar’s spouse. |
| (3) | Consists of 1,241,828 shares of our common stock and options to acquire 4,253 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007 held by Dr. Hofmann and 9,162 shares of our common stock held by Dr. Hofmann’s spouse. |
| (4) | Mr. Honigblum is the record owner of 349,390 shares of our common stock, assuming the exercise of currently exercisable preferred stock warrants which were issued to him at the request of Creation Capital LLC, which Creation Capital LLC was entitled to receive as partial compensation for the services it provided as our placement agent in connection with the completion of our offering of our preferred stock offerings. Mr. Honigblum is the Chief Executive Officer and a 50% co-owner of Creation Capital LLC and he joined our board of directors in December 2006. Mr. Honigblum is deemed to beneficially own 50% of the 19,633 shares of our common stock and an additional 29,227 shares of our common stock, assuming the exercise of currently exercisable preferred stock warrants, held by Creation Capital LLC. Mr. Honigblum disclaims beneficial ownership of the shares and warrants held by Creation Capital LLC except to the extent of his proportionate pecuniary interest therein. The shares noted above exclude 136,125 shares of our common stock held by Michael Morris, the President and the other 50% co-owner of Creation Capital LLC. |
| (5) | Consists of 205,060 shares of our common stock and options to acquire 326 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (6) | Consists of 15,706 shares of our common stock held by The Patel Family Trust U/A/D November 7, 1996, of which Mr. Patel and his spouse are the sole beneficiaries, and options held by Mr. Patel to acquire 35,666 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (7) | Consists of options to acquire 53,554 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (8) | Consists of 19,633 shares of our common stock and options to acquire 17,015 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (9) | Consists of 34,031 shares of our common stock held jointly by Dr. Dorr and his spouse. |
| (10) | Consists of options to acquire 21,312 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (11) | Consists of options to acquire 10,471 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (12) | Consists of options to acquire an aggregate of 9,161 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007. |
| (13) | See footnotes (2) through (12) except footnote 8. Also includes options to acquire an aggregate of 12,950 shares of our common stock currently exercisable or exercisable within 60 days after June 30, 2007, held by two executive officers not named in the table. |
| (14) |
Consists of 760,383 shares of our common stock and an additional 41,122 shares of our common stock, assuming the exercise of currently exercisable common stock warrants held by Vestal Venture Capital; 148,342 shares of our common stock and an additional 22,175 shares of our common stock, assuming the exercise of currently exercisable common stock warrants held by Lyonshare Venture Capital; and 5,236 shares of our common stock, assuming the exercise of currently exercisable common stock warrants held by Allan R. Lyons. Mr. Lyons is the managing member and sole owner of 21st Century Strategic Investment Planning, L.C., the general partner for both Vestal Venture Capital and Lyonshare Venture Capital. Mr. Lyons disclaims beneficial ownership of the shares held by Vestal Venture Capital and Lyonshare Venture Capital except to the extent of his proportionate pecuniary interest therein. |
110
Upon completion of this offering, we will be authorized to issue 40,000,000 shares of common stock, $0.01 par value per share, and 5,000,000 shares of preferred stock, $0.01 par value per share, and there will be 15,318,818 shares of common stock and no shares of preferred stock outstanding. Assuming the conversion of our preferred stock as of June 30, 2007, we had 10,668,818 shares of common stock outstanding held of record by 337 stockholders, and there were outstanding options to purchase 972,888 shares of common stock and outstanding warrants to acquire 1,062,067 shares of common stock, assuming the conversion of preferred stock warrants into common stock warrants. The following description summarizes the most important terms of our capital stock. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our amended and restated certificate of incorporation and amended and restated bylaws, effective upon completion of this offering, copies of which have been filed as exhibits to the registration statement, and to the applicable provisions of the Delaware General Corporation Law.
Common Stock
Holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, and do not have cumulative voting rights. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by our board of directors out of funds legally available for dividend payments. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common stock to be issued upon completion of this offering will be fully paid and nonassessable. The holders of common stock have no preferences or rights of conversion, exchange, pre-emption or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. In the event of any liquidation, dissolution or winding-up of our affairs, holders of common stock will be entitled to share ratably in our assets that are remaining after payment or provision for payment of all of our debts and obligations and after liquidation payments to holders of outstanding shares of preferred stock, if any.
Preferred Stock
Upon the closing of this offering, all outstanding shares of our preferred stock will have been converted into shares of our common stock. Following this offering, our amended and restated certificate of incorporation will be amended and restated to delete all reference to such shares of preferred stock. The preferred stock, if issued, would have priority over the common stock with respect to dividends and other distributions, including the distribution of assets upon liquidation. Our board of directors has the authority, without further stockholder authorization, to issue from time to time shares of preferred stock in one or more series and to fix the terms, limitations, relative rights and preferences and variations of each series. Although we have no present plans to issue any shares of preferred stock, the issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could decrease the amount of earnings and assets available for distribution to the holders of common stock, could adversely affect the rights and powers, including voting rights, of the common stock, and could have the effect of delaying, deterring or preventing a change in control of us or an unsolicited acquisition proposal.
Warrants
As of June 30, 2007 we had the following warrants outstanding to purchase a total of 1,062,067 shares of our common stock:
| • | A warrant to purchase in the aggregate 2,100,000 shares of Series A convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 549,745 shares of our common stock at an exercise price of $2.52 per share, terminating 2011. |
| • | A warrant to purchase in the aggregate 500,000 shares of Series B convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 130,893 shares of common stock at an exercise price of $5.04 per share, terminating 2011. |
111
| • | A warrant to purchase in the aggregate 1,203,750 shares of Series C convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 315,121 shares of our common stock at an exercise price of $8.40 per share, terminating 2013. |
| • | A warrant to purchase in the aggregate 253,290 shares of Series D convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 66,308 shares of common stock at an exercise price of $12.61 per share, terminating 2014. |
These warrants provide for adjustments of the exercise price and the number of shares underlying the warrants upon the occurrence of certain events, including stock dividends, stock splits, reclassifications or other changes in our corporate structure. The holders of these warrants have registration rights that are outlined below under the heading “Registration Rights.”
Registration Rights
The holders of 1,457,830 shares of common stock, assuming the conversion of our convertible preferred stock, and holders of 1,062,022 shares of common stock, assuming the exercise of preferred stock warrants and further assuming the conversion of such shares of convertible preferred stock, have entered into an agreement with us that provides certain registration rights to such holders and certain future transferees of their securities. These registration rights are subject to certain conditions and limitations, including our right, based on advice of the lead managing underwriter of a future offering, to limit the number of shares included in any such registration under certain circumstances. We are generally required to pay all expenses incurred in connection with registrations effected in connection with the registration rights below, excluding underwriting discounts and commissions. The registration rights described below with respect to these securities terminate on such date as the holders of such registrable securities become eligible to sell them under Rule 144 under the Securities Act.
Demand Rights. At any time after 180 days following the completion of our initial public offering, subject to specified limitations, holders of not less than a majority of then existing registrable securities may require that we effect the registration on Form S-1 or Form S-3 (or any other form we are qualified to use) of securities owned by such holders having an aggregate anticipated price to the public of at least $10,000,000 (before selling expenses), or at least $5,000,000 (before selling expenses) in the case of a Form S-3 registration, for sale under the Securities Act. We may be required to effect up to four such registrations in total. We may be required to effect up to two such registrations during the one-year period following the date holders initially notify us of their request that we effect such a registration. Holders of registrable securities who are not among the holders who initially request that we effect a registration are entitled to notice and are entitled to include their shares of common stock in the registration.
Shelf Registration Rights. At any time after we become eligible to file a registration statement on Form S-3, holders of not less than a majority of registrable securities may request, in writing, that we effect the registration on Form S-3, or any successor or similar short form, of securities having an aggregate anticipated offering price to the public of at least $10,000,000 (before selling expenses). We may be required to effect up to two such registrations during the one-year period following the date holders initially notify us of their request that we effect such a registration. Holders with these registration rights who are not among the holders who initially requested that we effect a registration are entitled to notice and are entitled to include their shares of common stock in the registration.
Piggyback Rights. If, at any time commencing 180 days following the completion of our initial public offering, we propose to register shares of our common stock under the Securities Act in connection with a public offering of common stock solely for cash, we will, prior to such filing, give written notice to all holders having registration rights of our intention to do so. Upon the written request of any holder or holders of registrable securities given to us in a timely manner, we shall cause all securities which we have been requested by such holder or holders to register to be registered under the Securities Act to the extent necessary to permit their sale
112
or other disposition in accordance with the intended methods of distribution specified in the request of the holder or holders. We shall have the right to withdraw any such registration without obligation to any stockholder, except for our obligation to pay all registration expenses related to such withdrawn registration. In addition, under certain circumstances, the underwriters, if any, may limit the number of shares included in any such registration. These piggyback registration rights do not apply to registrations of our securities that we initiate that are (i) incidental to any of our stock option plans or other employee benefit plans or a dividend reinvestment plan, (ii) incidental to a business combination or any other similar transaction, the purpose of which is not to raise capital, or (iii) pursuant to a so-called “unallocated” or “universal” shelf registration statement.
Effects of Anti-Takeover Provisions of Our Amended and Restated Certificate of Incorporation, Our Amended and Restated Bylaws and Delaware Law
The provisions of (1) Delaware law, (2) our amended and restated certificate of incorporation to be effective upon completion of this offering and (3) our amended and restated bylaws to be effective upon completion of this offering discussed below could discourage or make it more difficult to prevail in a proxy contest or effect other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or our best interests. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change of control of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. These provisions also may have the effect of preventing changes in our management.
Delaware Statutory Business Combinations Provision. We are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. For purposes of Section 203, a “business combination” is defined broadly to include a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and, subject to certain exceptions, an “interested stockholder” is a person who, together with his or her affiliates and associates, owns (or within three years prior, did own) 15% or more of the corporation’s voting stock.
Classified Board of Directors; Appointment of Directors to Fill Vacancies; Removal of Directors for Cause. Our amended and restated certificate of incorporation provides that our board of directors will be divided into three classes as nearly equal in number as possible. Each year the stockholders will elect the members of one of the three classes to a three-year term of office. All directors elected to our classified board of directors will serve until the election and qualification of their respective successors or their earlier resignation or removal. The board of directors is authorized to create new directorships and to fill any positions so created and is permitted to specify the class to which any new position is assigned. The person filling any of these positions would serve for the term applicable to that class. The board of directors (or its remaining members, even if less than a quorum) is also empowered to fill vacancies on the board of directors occurring for any reason for the remainder of the term of the class of directors in which the vacancy occurred. Members of the board of directors may only be removed for cause and only by the affirmative vote of holders of at least 75% of our outstanding voting stock. These provisions are likely to increase the time required for stockholders to change the composition of the board of directors. For example, in general, at least two annual meetings will be necessary for stockholders to effect a change in a majority of the members of the board of directors.
113
Authorization of Blank Check Preferred Stock. Our amended and restated certificate of incorporation provides that, upon completion of this offering, our board of directors will be authorized to issue, without stockholder approval, blank check preferred stock. Blank check preferred stock can operate as a defensive measure known as a “poison pill” by diluting the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors.
Advance Notice Provisions for Stockholder Proposals and Stockholder Nominations of Directors. Our amended and restated bylaws provide that, for nominations to the board of directors or for other business to be properly brought by a stockholder before a meeting of stockholders, the stockholder must first have given timely notice of the proposal in writing to our Secretary. For an annual meeting, a stockholder’s notice generally must be delivered not less than 45 days nor more than 75 days prior to the anniversary of the mailing date of the proxy statement for the previous year’s annual meeting. For a special meeting, the notice must generally be delivered no less than 60 days nor more than 90 days prior to the special meeting or ten days following the day on which public announcement of the meeting is first made. Detailed requirements as to the form of the notice and information required in the notice are specified in the amended and restated bylaws. If it is determined that business was not properly brought before a meeting in accordance with our bylaw provisions, this business will not be conducted at the meeting.
Special Meetings of Stockholders. Special meetings of the stockholders may be called only by our board of directors pursuant to a resolution adopted by a majority of the total number of directors.
No Stockholder Action by Written Consent. Our amended and restated certificate of incorporation does not permit our stockholders to act by written consent. As a result, any action to be effected by our stockholders must be effected at a duly called annual or special meeting of the stockholders.
Super-Majority Stockholder Vote required for Certain Actions. The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless the corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our amended and restated certificate of incorporation requires the affirmative vote of the holders of at least 75% of our outstanding voting stock to amend or repeal any of the provisions discussed in this section of this prospectus entitled “Effect of Anti-Takeover Provisions of Our Amended and Restated Certificate of Incorporation, Our Amended and Restated Bylaws and Delaware Law” or to reduce the number of authorized shares of common stock or preferred stock. This 75% stockholder vote would be in addition to any separate class vote that might in the future be required pursuant to the terms of any preferred stock that might then be outstanding. A 75% vote is also required for any amendment to, or repeal of, our amended and restated bylaws by the stockholders. Our amended and restated bylaws may be amended or repealed by a simple majority vote of the board of directors.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be American Stock Transfer and Trust Company.
Listing
At the present time, there is no established trading market for our common stock. We have applied to list our common stock on The NASDAQ Global Market under the symbol “AMCA.”
114
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common stock. Future sales of substantial amounts of our common stock in the public market, or the anticipation of such sales, could adversely affect market prices prevailing from time to time. Furthermore, because only a limited number of shares will be available for sale shortly after this offering due to existing contractual and legal restrictions on resale as described below, there may be sales of substantial amounts of our common stock in the public market after the restrictions lapse. This may adversely affect the prevailing market price and our ability to raise equity capital in the future.
Upon completion of this offering, we will have 15,318,818 shares of common stock outstanding, assuming the conversion of all outstanding shares of convertible preferred stock, no exercise of the underwriters’ over-allotment option and no exercise of any options and warrants outstanding as of March 31, 2007. Of these shares, all of the shares sold in this offering will be freely transferable without restriction or registration under the Securities Act, except for any shares purchased by one of our existing “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining shares of common stock are “restricted shares” as defined in Rule 144. Restricted shares may be sold in the public market only if registered or if they qualify for an exemption from registration under Rules 144, 144(k) or 701 of the Securities Act, as described below. Substantially all of these restricted shares will be subject to the 180-day lock-up period described below. Immediately after the 180-day lock-up period, 4,853,796 shares will be freely tradable under Rule 144(k) or Rule 701(g)(3) under the Securities Act and 4,433,768 shares will be eligible for resale under Rule 144 or Rule 701(g)(3), subject to volume limitations. 1,366,104 shares will be freely tradable or eligible for resale at various times after the 180-day lock-up period under Rule 144, Rule 144(k) or Rule 701(g)(3), some of which are subject to volume limitations. In addition, upon completion of this offering, a holder of warrants to acquire shares of our common stock will be able to net exercise such shares by surrendering a portion of that holder’s warrants as payment of the exercise price rather than paying the exercise price in cash. As of June 30, 2007 warrants to acquire approximately 449,579 and 546,137 shares of our common stock would be eligible to rely upon Rule 144(k) and Rule 144, respectively, if they are net exercised, subject to the lock-up agreements. The lock-up agreements may be extended or shortened in certain circumstances. Please see the section below entitled “Lock-up Agreements” for further information.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person, or persons whose shares are aggregated, who owns shares that were purchased from us, or any affiliate, at least one year previously, is entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| • | 1% of our then-outstanding shares of common stock, which will equal approximately shares immediately after this offering; or |
| • | the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a notice of the sale on Form 144. |
Sales under Rule 144 are also subject to manner of sale provisions, notice requirements and the availability of current public information about us. Rule 144 also provides that affiliates that sell our common stock that are not restricted securities must still comply with certain other restrictions of that rule on their manner of sale of our shares, other than the holding period requirement. We are unable to estimate the number of shares that will be sold under Rule 144 since this will depend on the market price for our common stock, the personal circumstances of the stockholder and other factors.
Rule 144(k)
Under Rule 144(k) as currently in effect, a person who is not deemed to have been one of our affiliates at any time during the 90 days preceding a sale, and who owns shares within the definition of “restricted securities” under Rule 144 that were purchased from us, or any affiliate, at least two years previously, would be entitled to sell shares under Rule 144(k) without regard to the volume limitations, manner of sale provisions, public information requirements or notice requirements described above.
115
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who purchased shares from us in connection with a qualified compensatory stock or option plan or other written agreement before the effective date of this offering is eligible to resell such shares 90 days after the effective date of this offering in reliance on Rule 144. Securities issued in reliance on Rule 701 are restricted securities and, subject to the contractual restrictions described above, beginning 90 days after the date of this prospectus, may be sold by persons other than “affiliates,” as defined in Rule 144, subject only to the manner of sale provisions of Rule 144 and by “affiliates” under Rule 144 without compliance with its one year minimum holding requirement.
Registration Rights
The holders of 1,457,830 shares of common stock, assuming the conversion of our convertible preferred stock, and holders of 1,062,022 shares of common stock, assuming the exercise of preferred stock warrants and further assuming the conversion of such shares of convertible preferred stock, have entered into an agreement with us that provides certain registration rights to these holders and certain future transferees of their securities. Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares held by affiliates. See “Description of Capital Stock—Registration Rights.”
Warrants
As of June 30, 2007, we had the following outstanding warrants to purchase a total of 1,062,067 shares of our common stock:
| • | A warrant to purchase in the aggregate 2,100,000 shares of Series A convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 549,745 shares of our common stock at an exercise price of $2.52 per share, terminating 2011. |
| • | A warrant to purchase in the aggregate 500,000 shares of Series B convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 130,893 shares of common stock at an exercise price of $5.04 per share, terminating 2011. |
| • | A warrant to purchase in the aggregate 1,203,750 shares of Series C convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 315,121 shares of our common stock at an exercise price of $8.40 per share, terminating 2013. |
| • | A warrant to purchase in the aggregate 253,290 shares of Series D convertible preferred stock which, upon completion of our initial public offering, will be converted to a warrant to purchase in the aggregate 66,308 shares of common stock at an exercise price of $12.61 per share, terminating 2014. |
All 1,062,067 shares of common stock issuable pursuant to these warrants are subject to lock-up agreements.
Stock Options
As of June 30, 2007, options to purchase a total of 972,888 shares of common stock were outstanding and exercisable. Substantially all of the shares subject to options are subject to lock-up agreements. As of June 30, 2007, an additional 99,099 shares of common stock were available for future option grants under our 2003 Stock Option Plan. As of the date of this prospectus, 2,000,000 shares of common stock will be reserved for future awards under the 2007 Stock Plan, which becomes effective upon completion of this offering.
116
Upon completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act covering all shares of common stock subject to outstanding options or issuable pursuant to our 2003 Stock Option Plan and 2007 Stock Plan. Subject to Rule 144 volume limitations applicable to affiliates, shares registered under any registration statements will be available for sale in the open market, except to the extent that the shares are subject to vesting restrictions with us or the contractual restrictions described below.
Lock-up Agreements
We, all of our officers, directors and substantially all of our stockholders have agreed, subject to limited exceptions, not to offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, file any registration statement with the SEC relating to the offering of any shares of our common stock or any securities convertible into or exercisable for shares of our common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock held prior to the offering during the period beginning on the date of this prospectus and ending 180 days thereafter, whether any such transaction is to be settled by delivery of our common stock or such other securities, cash or otherwise, without the prior written consent of Morgan Stanley & Co. Incorporated.
Morgan Stanley & Co. Incorporated may in its sole discretion choose to release any or all of these shares from these restrictions prior to the expiration of the 180-day period. The lock-up restrictions will not apply to transactions relating to common stock acquired in open market transactions after the closing of this offering provided that no filing under Section 16(a) of the Exchange Act is required or will be voluntarily made in connection with subsequent sales of common stock or other securities acquired in such market transactions. The lock-up restrictions also will not apply to certain transfers not involving a disposition for value, provided that the recipient agrees to be bound by these lock-up restrictions and provided that such transfers are not required to be reported in any public report or filing with the SEC, or otherwise, during the lock-up period.
The 180-day restricted period described above will be extended if:
| • | during the last 17 days of the 180-day restricted period, we issue an earnings release or disclose material news or a material event relating to our company occurs; or |
| • | prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day restricted period; |
in which case the restrictions described above will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release, the disclosure of the material news or the occurrence of the material event.
117
MATERIAL U.S. FEDERAL TAX CONSEQUENCES FOR
NON-U.S. HOLDERS OF COMMON STOCK
The following discussion is a general summary of the material U.S. federal income tax consequences of the ownership and disposition of our common stock applicable to “Non-U.S. Holders.” As used herein, a Non-U.S. Holder means a beneficial owner of our common stock that is neither a U.S. person nor a partnership for U.S. federal income tax purposes, and that will hold shares of our common stock as capital assets. For U.S. federal income tax purposes, a U.S. person includes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other business entity treated as a corporation for U.S. federal income tax purposes) created or organized in the United States or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is includible in gross income regardless of source; or |
| • | a trust that (A) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons, or (B) otherwise has validly elected to be treated as a U.S. domestic trust for U.S. federal income tax purposes. |
If a partnership (including an entity treated as a partnership for U.S. federal income tax purposes) holds shares of our common stock, the U.S. federal income tax treatment of each partner generally will depend on the status of the partner and the activities of the partnership and the partner. Partnerships acquiring our common stock, and partners in such partnerships, should consult their own tax advisors with respect to the U.S. federal income tax consequences of the ownership and disposition of our common stock.
This summary does not consider specific facts and circumstances that may be relevant to a particular Non-U.S. Holder’s tax position and does not consider U.S. state and local or non-U.S. tax consequences. It also does not consider Non-U.S. Holders subject to special tax treatment under the U.S. federal income tax laws (including partnerships or other pass-through entities, banks and insurance companies, dealers in securities, holders of our common stock held as part of a “straddle,” “hedge,” “conversion transaction” or other risk-reduction transaction, controlled foreign corporations, passive foreign investment companies, companies that accumulate earnings to avoid U.S. federal income tax, foreign tax-exempt organizations, former U.S. citizens or residents, persons who hold or receive common stock as compensation and persons subject to the alternative minimum tax). This summary is based on provisions of the Internal Revenue Code, applicable final, temporary and proposed U.S. Treasury regulations, administrative pronouncements of the U.S. Internal Revenue Service, or the IRS, and judicial decisions, all as in effect on the date hereof, and all of which are subject to change, possibly on a retroactive basis, and different interpretations.
This summary is included herein as general information only. Accordingly, each prospective Non-U.S. Holder is urged to consult its own tax advisor with respect to the U.S. federal, state, local and non-U.S. income, estate and other tax consequences of owning and disposing of our common stock.
U.S. Trade or Business Income
For purposes of this discussion, dividend income and gain on the sale or other taxable disposition of our common stock will be considered to be “U.S. trade or business income” if such income or gain is (i) effectively connected with the conduct by a Non-U.S. Holder of a trade or business within the United States and (ii) in the case of a Non-U.S. Holder that is eligible for the benefits of an income tax treaty with the United States, attributable to a permanent establishment (or, for an individual, a fixed base) maintained by the Non-U.S. Holder in the United States. Generally, U.S. trade or business income is not subject to U.S. federal withholding tax (provided the Non-U.S. Holder complies with applicable certification and disclosure requirements); instead, U.S. trade or business income is subject to U.S. federal income tax on a net income basis at regular U.S. federal
118
income tax rates in the same manner as a U.S. person, unless an applicable income tax treaty provides otherwise. Any U.S. trade or business income received by a corporate Non-U.S. holder may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
Dividends
Distributions of cash or property that we pay will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). A Non-U.S. Holder generally will be subject to U.S. federal withholding tax at a 30% rate, or, if the Non-U.S. Holder is eligible, at a reduced rate prescribed by an applicable income tax treaty, on any dividends received in respect of our common stock. If the amount of a distribution exceeds our current and accumulated earnings and profits, such excess first will be treated as a tax-free return of capital to the extent of the Non-U.S. Holder’s tax basis in our common stock (with a corresponding reduction in such Non-U.S. Holder’s tax basis in our common stock), and thereafter will be treated as capital gain. In order to obtain a reduced rate of U.S. federal withholding tax under an applicable income tax treaty, a Non-U.S. Holder will be required to provide a properly executed IRS Form W-8BEN certifying under penalties of perjury its entitlement to benefits under the treaty. Special certification requirements and other requirements apply to certain Non-U.S. Holders that are entities rather than individuals. A Non-U.S. Holder of our common stock that is eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by filing an appropriate claim for a refund with the IRS on a timely basis. A Non-U.S. Holder should consult its own tax advisor regarding its possible entitlement to benefits under an income tax treaty and the filing of a U.S. tax return for claiming a refund of U.S. federal withholding tax.
The U.S. federal withholding tax does not apply to dividends that are U.S. trade or business income, as defined and discussed above, of a Non-U.S. Holder who provides a properly executed IRS Form W-8ECI, certifying under penalties of perjury that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States.
Dispositions of Our Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax in respect of any gain on a sale or other disposition of our common stock unless:
| • | the gain is U.S. trade or business income, as defined and discussed above; |
| • | the Non-U.S. Holder is an individual who is present in the United States for 183 or more days in the taxable year of the disposition and meets other conditions; or |
| • | we are or have been a “U.S. real property holding corporation,” or a USRPHC, under section 897 of the Internal Revenue Code at any time during the shorter of the five year period ending on the date of disposition and the Non-U.S. Holder’s holding period for our common stock. |
In general, a corporation is a USRPHC if the fair market value of its “U.S. real property interests” (as defined in the Internal Revenue Code and applicable Treasury regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business. If we are determined to be a USRPHC, the U.S. federal income and withholding taxes relating to interests in USRPHCs nevertheless will not apply to gains derived from the sale or other disposition of our common stock by a Non-U.S. Holder whose shareholdings, actual and constructive, at all times during the applicable period, amount to 5% or less of our common stock, provided that our common stock is regularly traded on an established securities market, within the meaning of the applicable Treasury regulations. We are not currently a USRPHC, and we do not anticipate becoming a USRPHC in the future. However, no assurance can be given that we will not be a USRPHC, or that our common stock will be considered regularly traded on an established securities market, when a Non-U.S. Holder sells its shares of our common stock.
119
Information Reporting and Backup Withholding Requirements
We must annually report to the IRS and to each Non-U.S. Holder any dividend income that is subject to U.S. federal withholding tax, or that is exempt from such withholding tax pursuant to an income tax treaty. Copies of these information returns also may be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides. Under certain circumstances, the Internal Revenue Code imposes a backup withholding obligation (currently at a rate of 28%) on certain reportable payments. Dividends paid to a Non-U.S. Holder of our common stock generally will be exempt from backup withholding if the Non-U.S. Holder provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption.
The payment of the proceeds from the disposition of our common stock to or through the U.S. office of any broker, U.S. or foreign, will be subject to information reporting and possible backup withholding unless the holder certifies as to its non-U.S. status under penalties of perjury or otherwise establishes an exemption, provided that the broker does not have actual knowledge or reason to know that the holder is a U.S. person or that the conditions of any other exemption are not, in fact, satisfied. The payment of the proceeds from the disposition of our common stock to or through a non-U.S. office of a non-U.S. broker is one that will not be subject to information reporting or backup withholding unless the non-U.S. broker has certain types of relationships with the United States (a “U.S. related person”). In the case of the payment of the proceeds from the disposition of our common stock to or through a non-U.S office of a broker that is either a U.S. person or a U.S. related person, the Treasury regulations require information reporting (but not backup withholding) on the payment unless the broker has documentary evidence in its files that the holder is a Non-U.S. Holder and the broker has no knowledge to the contrary. Non-U.S. Holders should consult their own tax advisors on the application of information reporting and backup withholding to them in their particular circumstances (including upon their disposition of our common stock).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder will be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, if the Non-U.S. Holder provides the required information to the IRS on a timely basis. Non-U.S. Holders should consult their own tax advisors regarding the filing of a U.S. tax return for claiming a refund of such backup withholding.
120
Under the terms and subject to the conditions contained in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Morgan Stanley & Co. Incorporated, Jefferies & Company, Inc. and CIBC World Markets Corp. are acting as representatives, have severally agreed to purchase, and we have agreed to sell to them, the number of shares of common stock indicated in the table below:
| Name |
Number of Shares | |
| Morgan Stanley & Co. Incorporated |
||
| Jefferies & Company, Inc. |
||
| CIBC World Markets Corp. |
||
| Total |
4,650,000 | |
The underwriters are offering the shares of common stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to other conditions. The underwriters are obligated to take and pay for all of the shares of common stock offered by this prospectus if any shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
The underwriters initially propose to offer part of the shares of common stock directly to the public at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions, and part to certain dealers at a price that represents a concession not in excess of $ a share under the public offering price. No underwriter may allow, and no dealer may re-allow, any concession to other underwriters or to certain dealers. After the initial offering of the shares of common stock, the offering price and other selling terms may from time to time be varied by the representatives.
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of 697,500 additional shares of common stock at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares of common stock offered by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase approximately the same percentage of the additional shares of common stock as the number listed next to the underwriter’s name in the preceding table bears to the total number of shares of common stock listed next to the names of all underwriters in the preceding table. If the underwriters’ over-allotment option is exercised in full, the total price to the public would be $ , the total underwriters’ discounts and commissions would be $ and the total proceeds to us would be $ .
The following table shows the per share and total underwriting discounts and commissions that we are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option.
| No Exercise | Full Exercise | |||||
| Per share |
$ | $ | ||||
| Total |
$ | $ | ||||
In addition, we estimate that the expenses of this offering other than underwriting discounts and commissions payable by us will be approximately $2,750,000.
The underwriters have informed us that they do not intend to make sales to accounts over which they exercise discretionary authority in excess of 5% of the total number of shares of common stock offered by them.
121
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “AMCA.”
We, all of our directors and officers and holders of substantially all our outstanding stock and securities exercisable for or convertible into shares of common stock have agreed that, subject to certain limitations, without the prior written consent of Morgan Stanley & Co. Incorporated on behalf of the underwriters, we and they will not, during the period beginning on the date of this prospectus and ending 180 days thereafter:
| • | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock; |
| • | file any registration statement with the SEC relating to the offering of any shares of our common stock or any securities convertible into or exercisable for shares of our common stock; or |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock; |
whether any transaction described above is to be settled by delivery of our common stock or such other securities, in cash or otherwise.
Moreover, the 180-day restricted period described in the preceding paragraph will be extended if:
| • | during the last 17 days of the 180-day restricted period, we issue an earnings release or disclose material news or a material event relating to our company occurs; or |
| • | prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day restricted period; |
in which case the restrictions described in the immediately preceding sentence will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release, the disclosure of the material news or the occurrence of the material event.
The restrictions described in the immediately preceding two paragraphs do not apply to:
| • | the sale of shares to the underwriters; |
| • | the issuance by us of shares of common stock upon the exercise of an option or a warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing; or |
| • | transactions by any person other than us relating to shares of common stock or other securities acquired in open market transactions after the completion of this offering of shares. |
At our request, the underwriters have reserved for sale at the initial public offering price up to 5% of the shares offered hereby for officers, directors, employees and certain other persons associated with us. The number of shares available for sale to the general public will be reduced to the extent such persons purchase such reserved shares. Any reserved shares not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered hereby. Any shares purchased through this directed share program will be subject to an agreement between the purchaser of the shares and Morgan Stanley & Co. Incorporated, providing that the shares may not be sold, transferred or otherwise disposed of as described in the preceding paragraphs. The directed share program is being arranged through Morgan Stanley & Co. Incorporated.
In order to facilitate this offering of common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the common stock. Specifically, the underwriters may sell
122
more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the over-allotment option. The underwriters can close out a covered short sale by exercising the over-allotment option or by purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the over-allotment option. The underwriters may also sell shares in excess of the over-allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in this offering. In addition, to stabilize the price of the common stock, the underwriters may bid for and purchase shares of common stock in the open market. Finally, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the common stock in the offering if the syndicate repurchases previously distributed common stock to cover syndicate short positions or to stabilize the price of the common stock. These activities may raise or maintain the market price of the common stock above independent market levels or prevent or retard a decline in the market price of the common stock. The underwriters are not required to engage in these activities and may end any of these activities at any time.
The underwriters may in the future provide investment banking services to us for which they would receive customary compensation.
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each a “Relevant Member State”), each underwriter has represented and agreed that it has not made and will not make an offer to the public of any shares of common stock in that Relevant Member State, except that it may make an offer to the public of shares of common stock in that Relevant Member State at any time under the following exemptions under the Prospectus Directive:
| • | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| • | to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| • | an offer addressed to fewer than 100 natural or legal persons in that Relevant Member State (other than qualified investors); or |
| • | in any other circumstances which do not require the publication by us of a prospectus pursuant to Article 3(2) of the Prospectus Directive. |
For the purposes of the above, the expression an “offer to the public” in relation to any shares of common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares of common stock to be offered so as to enable an investor to decide to purchase or subscribe the shares of common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State and the expression “qualified investor” has the meaning set forth in Article 2(1) of the Prospectus Directive.
Each underwriter has represented and agreed that it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000) in connection with the issue or sale of the common stock in circumstances in which Section 21(1) of such Act does not apply to us
123
and it has complied and will comply with all applicable provisions of such Act with respect to anything done by it in relation to any shares of common stock in, from or otherwise involving the United Kingdom.
Pricing of the Offering
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. Among the factors to be considered in determining the initial public offering price will be our future prospects and those of our industry in general; sales, earnings and other financial operating information in recent periods; and the price-earnings ratios, price-sales ratios and market prices of securities and certain financial and operating information of companies engaged in activities similar to ours. The estimated initial public offering price range set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters. The underwriters may agree to allocate a number of shares to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters that may make Internet distributions on the same basis as other allocations.
The validity of the issuance of the common stock offered by us in this offering will be passed upon for us by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts. The underwriters are being represented by Davis Polk & Wardwell, Menlo Park, California.
Ernst & Young LLP, independent registered public accounting firm, has audited our financial statements at December 31, 2005 and 2006 and for each of the three years in the period ended December 31, 2006, as set forth in their report. We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
124
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
You may read and copy all or any portion of the registration statement without charge at the public reference room of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of the registration statement may be obtained from the SEC at prescribed rates from the public reference room of the SEC at such address. You may obtain information regarding the operation of the public reference room by calling 1-800-SEC-0330. In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, accordingly, will file annual reports containing financial statements audited by an independent public accounting firm, quarterly reports containing unaudited financial data, current reports, proxy statements and other information with the SEC. You will be able to inspect and copy such periodic reports, proxy statements and other information at the SEC’s public reference room, and the web site of the SEC referred to above. We will also maintain a web site at http://www.amedicacorp.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our web site is not part of this prospectus.
125
(A Development Stage Company)
| F-2 | ||
| F-3 | ||
| F-4 | ||
| Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) |
F-5 | |
| F-7 | ||
| F-8 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
Amedica Corporation
We have audited the accompanying balance sheets of Amedica Corporation (a development stage company) as of December 31, 2005 and 2006, and the related statements of operations, convertible preferred stock and stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2006. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Amedica Corporation (a development stage company) at December 31, 2005 and 2006, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006, in conformity with U.S. generally accepted accounting principles.
As discussed in Notes 1 and 7 to the financial statements, Amedica Corporation (a development stage company) changed its method of accounting for stock-based compensation in accordance with guidance provided in Statement of Financial Accounting Standard No. 123(R), Share-Based Payment during the year ended December 31, 2006.
Ernst & Young LLP
Salt Lake City, Utah
May 18, 2007, except for Note 12
as to which the date is July 26, 2007
F-2
(A Development Stage Company)
BALANCE SHEETS
| December 31, | March 31, 2007 |
Pro Forma 2007 |
||||||||||||||
| 2005 | 2006 | |||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| ASSETS | ||||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 1,252,570 | $ | 1,689,135 | $ | 2,045,322 | $ | 2,045,322 | ||||||||
| Marketable securities |
4,927,251 | 11,780,000 | 8,280,000 | 8,280,000 | ||||||||||||
| Accrued interest receivable |
52,240 | 49,396 | 27,604 | 27,604 | ||||||||||||
| Prepaid expenses |
18,550 | 45,846 | 45,846 | 45,846 | ||||||||||||
| Total current assets |
6,250,611 | 13,564,377 | 10,398,772 | 10,398,772 | ||||||||||||
| Property and equipment, net |
1,370,278 | 4,057,090 | 4,534,990 | 4,534,990 | ||||||||||||
| Other assets: |
||||||||||||||||
| Deferred offering costs |
115,297 | 1,314,588 | 1,348,952 | 1,348,952 | ||||||||||||
| Restricted cash |
100,000 | 850,000 | 850,000 | 850,000 | ||||||||||||
| Deposits |
151,508 | 75,255 | 75,255 | 75,255 | ||||||||||||
| Total other assets |
366,805 | 2,239,843 | 2,274,207 | 2,274,207 | ||||||||||||
| Total assets |
$ | 7,987,694 | $ | 19,861,310 | $ | 17,207,969 | $ | 17,207,969 | ||||||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||||||||||
| Current liabilities: |
||||||||||||||||
| Accounts payable |
$ | 207,216 | $ | 975,162 | $ | 241,825 | $ | 241,825 | ||||||||
| Accrued liabilities |
175,623 | 142,968 | 91,954 | 91,954 | ||||||||||||
| Deferred rent |
12,910 | 3,063 | 6,338 | 6,338 | ||||||||||||
| Deferred revenue |
— | 98,933 | 98,933 | 98,933 | ||||||||||||
| Current portion of long-term debt |
— | 376,539 | 418,616 | 418,616 | ||||||||||||
| Total current liabilities |
|
395,749 |
|
|
1,596,665 |
|
|
857,666 |
|
857,666 | ||||||
| Deferred rent |
2,506 | 79,922 | 77,699 | 77,699 | ||||||||||||
| Deferred revenue |
98,933 | — | — | — | ||||||||||||
| Preferred stock warrant liability |
1,404,000 | 2,623,550 | 6,304,963 | — | ||||||||||||
| Long-term debt |
— | 1,245,359 | 1,137,625 | 1,137,625 | ||||||||||||
| Commitments and contingencies |
||||||||||||||||
| Convertible preferred stock, $0.01 par value, 40,000,000 shares authorized; 19,000,058 shares issued and outstanding at December 31, 2005 and 27,400,058 shares issued and outstanding at December 31, 2006 and March 31, 2007 (aggregate liquidation value of $14,400,035 at December 31, 2005, and $31,200,035 at December 31, 2006 and March 31, 2007); no shares issued and outstanding pro forma |
|
12,153,095 |
|
|
26,389,982 |
|
|
26,389,982 |
|
— | ||||||
| Stockholders’ equity (deficit): |
||||||||||||||||
| Common stock, $0.01 par value, 60,000,000 shares authorized; |
|
22,658 |
|
|
22,719 |
|
|
22,719 |
|
|
94,447 |
| ||||
| Additional paid-in capital |
|
379,996 |
|
|
514,705 |
|
|
591,632 |
|
|
33,214,849 |
| ||||
| Accumulated other comprehensive loss |
(21,205 | ) | — | — | — | |||||||||||
| Deficit accumulated during the development stage |
(6,448,038 | ) | (12,611,592 | ) | (18,174,317 | ) | (18,174,317 | ) | ||||||||
| Total stockholders’ equity (deficit) |
(6,066,589 | ) | (12,074,168 | ) | (17,559,966 | ) | 15,134,979 | |||||||||
| Total liabilities, convertible preferred stock and stockholders’ equity (deficit) |
$ | 7,987,694 | $ | 19,861,310 | $ | 17,207,969 | $ | 17,207,969 | ||||||||
The accompanying notes are an integral part of these financial statements.
F-3
(A Development Stage Company)
STATEMENTS OF OPERATIONS
| For the years ended December 31, |
Three months ended March 31, |
For the period from December 10, |
||||||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
| Grant revenue |
$ | 208,252 | $ | 69,207 | $ | 94,850 | $ | — | $ | — | $ | 1,234,476 | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
1,419,293 | 2,966,991 | 4,974,380 |
|
1,100,125 |
|
|
1,479,340 |
|
|
11,802,694 |
| ||||||||||||
| General and administrative |
398,208 | 576,295 | 1,113,500 |
|
184,425 |
|
|
405,380 |
|
|
2,806,322 |
| ||||||||||||
| Sales and marketing |
— | 416,847 | 607,538 | 111,038 | 125,740 | 1,150,125 | ||||||||||||||||||
| Total operating expenses |
1,817,501 | 3,960,133 | 6,695,418 | 1,395,588 | 2,010,460 | 15,759,141 | ||||||||||||||||||
| Loss from operations |
(1,609,249 | ) | (3,890,926 | ) | (6,600,568 | ) | (1,395,588 | ) | (2,010,460 | ) | (14,524,665 | ) | ||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Interest income |
107,211 | 248,838 | 805,437 | 150,487 | 166,870 | 1,330,365 | ||||||||||||||||||
| Interest expense |
— | — | (77,498 | ) | — | (37,722 | ) | (176,590 | ) | |||||||||||||||
| Change in value of preferred stock warrants |
(254,089 | ) | (577,000 | ) | (290,925 | ) | (72,731 | ) | (3,681,413 | ) | (4,803,427 | ) | ||||||||||||
| Total other income (expense) |
(146,878 | ) | (328,162 | ) |
|
437,014 |
|
77,756 | (3,552,265 | ) | (3,649,652 | ) | ||||||||||||
| Net loss |
$ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,317,832 | ) | $ | (5,562,725 | ) | $ | (18,174,317 | ) | ||||||
| Basic and diluted net loss per share |
$ | (0.78 | ) | $ | (1.87 | ) | $ | (2.72 | ) | $ | (0.58 | ) | $ | (2.45 | ) | |||||||||
| Shares used to compute basic and diluted net loss per share |
2,247,611 | 2,254,454 | 2,267,464 | 2,265,863 | 2,271,986 | |||||||||||||||||||
| Pro forma net loss per share (unaudited) |
$ | (0.68 | ) | $ | (0.59 | ) | ||||||||||||||||||
| Shares used to compute pro forma basic and diluted net loss per share (unaudited) |
9,108,798 | 9,444,682 | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-4
(A Development Stage Company)
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
| Convertible Preferred Stock |
Common Stock | Additional Capital |
Accumulated Income (loss) |
Deficit Stage |
Total Equity (Deficit) |
|||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
| Issuance of common stock to founders for cash at $0.000095 per share on December 10, 1996 (inception) |
— | $ | — | 2,094,240 | $ | 20,942 | $ | (20,742 | ) | $ | — | $ | — | $ | 200 | |||||||||||||
| Net loss |
— | — | — | — | — | — | (8,810 | ) | (8,810 | ) | ||||||||||||||||||
| Balance at December 31, 1996 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (8,810 | ) | (8,610 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | (21,143 | ) | (21,143 | ) | ||||||||||||||||||
| Balance at December 31, 1997 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (29,953 | ) | (29,753 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | (40,967 | ) | (40,967 | ) | ||||||||||||||||||
| Balance at December 31, 1998 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (70,920 | ) | (70,720 | ) | |||||||||||||||||
| Net income |
— | — | — | — | — | — | 8,324 | 8,324 | ||||||||||||||||||||
| Balance at December 31, 1999 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (62,596 | ) | (62,396 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | (87,300 | ) | (87,300 | ) | ||||||||||||||||||
| Balance at December 31, 2000 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (149,896 | ) | (149,696 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | (105,278 | ) | (105,278 | ) | ||||||||||||||||||
| Balance at December 31, 2001 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (255,174 | ) | (254,974 | ) | |||||||||||||||||
| Net income |
— | — | — | — | — | — | 12,779 | 12,779 | ||||||||||||||||||||
| Balance at December 31, 2002 |
— | — | 2,094,240 | 20,942 | (20,742 | ) | — | (242,395 | ) | (242,195 | ) | |||||||||||||||||
| Issuance of common stock in exchange for shareholder note payable and interest at $2.29 per share in October 2003 |
— | — | 129,263 | 1,293 | 294,978 | — | — | 296,271 | ||||||||||||||||||||
| Issuance of Series A convertible preferred stock for cash at $0.60 per share in November 2003 for cash, net of offering costs |
10,195,040 | 5,289,266 | — | — | — | — | — | |||||||||||||||||||||
| Compensation expense related to stock options granted to consultants |
— | — | — | — | 3,094 | — | — | 3,094 | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (230,428 | ) | (230,428 | ) | ||||||||||||||||||
| Balance at December 31, 2003 |
10,195,040 | 5,289,266 | 2,223,503 | 22,235 | 277,330 | — | (472,823 | ) | (173,258 | ) | ||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 24,924 | 249 | 23,533 | — | — | 23,782 | ||||||||||||||||||||
| Issuance of Series A convertible preferred stock for cash at $0.60 per share in January 2004, net of offering costs |
3,805,018 |
|
1,666,405 |
— | — | — | — | — | — | |||||||||||||||||||
| Issuance of Series B convertible preferred stock for cash at $1.20 per share in November 2004, net of offering costs |
5,000,000 |
|
5,197,424 |
— | — | — | — | — | — | |||||||||||||||||||
| Compensation expense related to stock options granted to consultants |
— | — | — | — | 18,391 | — | — | 18,391 | ||||||||||||||||||||
| Unrealized loss on marketable securities |
— | — | — | — | — | (15,525 | ) | — | (15,525 | ) | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (1,756,127 | ) | (1,756,127 | ) | ||||||||||||||||||
| Comprehensive loss |
(1,771,652 | ) | ||||||||||||||||||||||||||
| Balance at December 31, 2004 |
19,000,058 | $ | 12,153,095 | 2,248,427 | $ | 22,484 | $ | 319,254 | $ | (15,525 | ) | $ | (2,228,950 | ) | $ | (1,902,737 | ) | |||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
AMEDICA CORPORATION
(A Development Stage Company)
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
| Convertible Preferred Stock |
Common Stock | Additional Capital |
Accumulated Income (loss) |
Deficit Stage |
Total Equity (Deficit) |
||||||||||||||||||||||
| Shares | Amount | Shares | Amount | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | $ | — | 17,434 |
$ |
174 |
$ |
16,476 |
$ | — | $ | — | $ | 16,650 | |||||||||||||
| Compensation expense related to stock options granted to consultants |
— | — | — | — | 44,266 | — | — | 44,266 | |||||||||||||||||||
| Unrealized loss on marketable securities |
— | — | — | — | — | (5,680 | ) | — | (5,680 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | (4,219,088 | ) | (4,219,088 | ) | |||||||||||||||||
| Comprehensive loss |
(4,224,768 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2005 |
19,000,058 | 12,153,095 | 2,265,861 |
|
22,658 |
|
379,996 |
(21,205 | ) | (6,448,038 | ) | (6,066,589 | ) | ||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 6,125 |
|
61 |
|
3,539 |
— | — | 3,600 | |||||||||||||||||
| Issuance of Series C convertible preferred stock for cash at $2.00 per share in February 2006, net of offering costs |
8,400,000 |
|
14,236,887 |
— | — | — | — | — | — | ||||||||||||||||||
| Compensation expense related to stock options granted to employees and consultants |
— | — | — | — | 131,170 | — | — | 131,170 | |||||||||||||||||||
| Decrease in unrealized loss on marketable securities |
— | — | — | — | — | 21,205 | — | 21,205 | |||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (6,163,554 | ) | (6,163,554 | ) | |||||||||||||||||
| Comprehensive loss |
(6,142,349 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2006 |
27,400,058 | $ | 26,389,982 | 2,271,986 |
|
22,719 |
|
514,705 |
— | (12,611,592 | ) | (12,074,168 | ) | ||||||||||||||
| Compensation expense related to stock options granted to employees and consultants (unaudited) |
— | — | — | — | 76,927 | — | — | 76,927 | |||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | — | (5,562,725 | ) | (5,562,725 | ) | |||||||||||||||||
| Comprehensive loss (unaudited) |
(5,562,725 | ) | |||||||||||||||||||||||||
| Balance at March 31, 2007 (unaudited) |
27,400,058 | $ | 26,389,982 | 2,271,986 |
$ |
22,719 |
$ |
591,632 |
$ | — | $ | (18,174,317 | ) | $ | (17,559,966 | ) | |||||||||||
The accompanying notes are an integral part of these financial statements.
F-6
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
| For the years ended December 31, |
Three Months Ended March 31, |
For the period from December 10, 1996 (inception) to March 31, 2007 |
||||||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||
| (unaudited) |
(unaudited) | |||||||||||||||||||||||
| Cash Flows from Operating Activities: |
||||||||||||||||||||||||
| Net loss |
$ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,317,832 | ) | $ | (5,562,725 | ) | $ | (18,174,317 | ) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||||||||||
| Depreciation and amortization |
32,487 | 164,374 | 546,100 | 49,513 | 237,551 | 985,135 | ||||||||||||||||||
| Loss on impairment of assets |
— | — | — | — | — | 20,935 | ||||||||||||||||||
| Amortization of premiums on marketable securities |
29,962 | 75,075 | 18,212 | 10,464 | — | 123,249 | ||||||||||||||||||
| Interest on shareholder loan |
— | — | — | — | — | 61,370 | ||||||||||||||||||
| Stock based compensation |
18,391 | 44,266 | 131,170 | 25,474 | 76,927 | 273,848 | ||||||||||||||||||
| Revaluation of preferred stock warrant liability |
|
254,089 |
|
577,000 |
|
290,925 |
|
|
72,731 |
|
3,681,413 |
|
4,803,427 |
| ||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||||||
| Interest receivable |
(61,359 | ) | 9,119 | 2,844 | 10,575 | 21,792 | (27,604 | ) | ||||||||||||||||
| Prepaid expenses |
(42,909 | ) | 60,519 | (27,296 | ) | 90,696 | — | (45,846 | ) | |||||||||||||||
| Deferred offering costs |
— | (115,297 | ) | (1,199,291 | ) | 115,297 | (34,364 | ) | (1,348,952 | ) | ||||||||||||||
| Deposits |
(74,000 | ) | (77,508 | ) | 76,253 | 76,252 | — | (75,255 | ) | |||||||||||||||
| Accounts payable and accrued liabilities |
203,514 | (3,827 | ) | 735,291 | 111,241 | (784,351 | ) | 333,779 | ||||||||||||||||
| Deferred rent |
18,767 | (3,351 | ) | 67,569 | (5,842 | ) | 1,052 | 84,037 | ||||||||||||||||
| Deferred revenue |
96,000 | 2,933 | — | — | — | 98,933 | ||||||||||||||||||
| Net cash used in operating activities |
(1,281,185 | ) | (3,485,785 | ) | (5,521,777 | ) | (761,431 | ) | (2,362,705 | ) | (12,887,261 | ) | ||||||||||||
| Cash Flows from Investing Activities: |
||||||||||||||||||||||||
| Purchases of property and equipment |
(694,556 | ) | (854,088 | ) | (3,232,912 | ) | (1,002,168 | ) | (715,451 | ) | (5,541,060 | ) | ||||||||||||
| Purchases of marketable securities |
(8,516,594 | ) | (5,193,165 | ) | (30,097,756 | ) | (15,262,044 | ) | — | (43,807,515 | ) | |||||||||||||
| Increase in restricted cash |
(100,000 | ) |
|
— |
|
(750,000 | ) | — | — | (850,000 | ) | |||||||||||||
| Maturities of marketable securities |
— | 8,656,266 | 23,248,000 | 2,200,000 | 3,500,000 | 35,404,266 | ||||||||||||||||||
| Net cash provided by (used in) investing activities: |
(9,311,150 | ) | 2,609,013 | (10,832,668 | ) | (14,064,212 | ) | 2,784,549 | (14,794,309 | ) | ||||||||||||||
| Cash Flows from Financing Activities: |
||||||||||||||||||||||||
| Proceeds from shareholder note payable |
— | — | — | — | — | 234,901 | ||||||||||||||||||
| Proceeds from issuance of convertible preferred stock and warrants, net of issuance costs |
7,436,740 | — | 15,165,512 | 15,165,512 | — | 27,891,518 | ||||||||||||||||||
| Proceeds from issuance of common stock |
23,782 | 16,650 | 3,600 | — | — | 44,232 | ||||||||||||||||||
| Proceeds from long-term debt |
— | — | 1,621,898 | — | — | 1,621,898 | ||||||||||||||||||
| Repayments of long-term debt |
— | — | — | — | (65,657 | ) | (65,657 | ) | ||||||||||||||||
| Net cash provided by (used in) financing activities |
7,460,522 | 16,650 | 16,791,010 | 15,165,512 | (65,657 | ) | 29,726,892 | |||||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
(3,131,813 | ) | (860,122 | ) | 436,565 | 339,869 | 356,187 |
|
2,045,322 |
| ||||||||||||||
| Cash and cash equivalents at beginning of period |
5,244,505 | 2,112,692 | 1,252,570 | 1,252,570 | 1,689,135 | — | ||||||||||||||||||
| Cash and cash equivalents at end of period |
$ | 2,112,692 | $ | 1,252,570 | $ | 1,689,135 | $ | 1,592,439 | $ | 2,045,322 | $ | 2,045,322 | ||||||||||||
| Noncash financing activities: |
||||||||||||||||||||||||
| Common stock issued in exchange for shareholder note and accrued interest |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | 296,271 | ||||||||||||
| Warrants issued in connection with preferred stock offerings |
572,911 | — | 928,625 | 928,625 | — | 1,501,536 | ||||||||||||||||||
| Supplemental cash flow information |
||||||||||||||||||||||||
| Cash paid for interest |
$ | — | $ | — | $ | 76,574 | $ | — | $ | 37,722 | $ | 114,296 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
F-7
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
1. Organization and Summary of Significant Accounting Policies
Amedica Corporation (“Amedica” or the “Company”), incorporated in the state of Delaware on December 10, 1996, is a development stage orthopedic implants company focusing on using silicon nitride ceramic technologies to develop, manufacture and commercialize a broad range of advanced, high-performance spine and joint implants.
The Company is considered a development stage company as planned principal operations have not yet commenced and the revenue generated from research grants did not constitute significant and sustained revenue. Since inception, the Company has devoted substantially all of its resources to start-up activities, raising capital, research and development, and build-out of its ceramics manufacturing facility.
Unaudited Interim Financial Information
The accompanying balance sheet as of March 31, 2007, and the related statements of operations and cash flows for the three month periods ended March 31, 2006 and 2007 and for the period from December 10, 1996 (inception) to March 31, 2007 and the statement of convertible preferred stock and stockholders’ equity (deficit) for the three months ended March 31, 2007 and related information contained in the notes to financial statements are unaudited. These unaudited financial statements and notes have been prepared in accordance with U.S. generally accepted accounting principles. In the opinion of the Company’s management, the unaudited financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, consisting of only normal recurring adjustments, necessary for the fair presentation of the Company’s financial position, results of operations and cash flows for the three months ended March 31, 2006 and 2007 and for the period from December 10, 1996 (inception) to March 31, 2007. The results for the three months ended March 31, 2007 are not necessarily indicative of the results of operations to be expected for the year ending December 31, 2007 or for any other interim period or future year.
Unaudited Pro Forma Balance Sheet
In May 2007, the board of directors authorized management of the Company to file a registration statement with the Securities and Exchange Commission permitting the Company to sell shares of its common stock to the public. The unaudited pro forma balance sheet as of March 31, 2007 and pro forma basic and diluted net loss per share reflect the automatic conversion of all of the Series A, Series B and Series C convertible preferred stock outstanding at the time of the offering into 7,172,696 shares of common stock upon the closing of the Company’s initial public offering, and the impact of the reclassification of the preferred stock warrant liability into additional paid in capital as a result of the automatic conversion of warrants to purchase preferred stock into warrants to purchase common stock upon closing of the Company’s initial public offering.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
F-8
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Fair Value of Financial Instruments
Financial instruments, including cash and cash equivalents, marketable securities, and restricted cash are carried at fair value. Other financial instruments, including other current assets, accounts payable and accrued liabilities are carried at cost, which management believes approximates fair value given their short-term nature.
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash, cash equivalents, marketable securities and restricted cash. The Company limits its exposure to credit loss by placing its cash with high credit-quality financial institutions. The Company has established guidelines relative to diversification of its cash and investment securities and their maturities that are intended to secure safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates and changes in the Company’s operations and financial position.
Cash, Cash Equivalents and Marketable Securities
The Company invests its available cash balances in bank deposits, money market funds, U.S. government securities and other investment grade debt securities that have strong credit ratings. The Company considers all highly liquid investments with an original maturity of three months or less at the time of purchase to be cash equivalents.
The Company accounts for its investments in marketable securities in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 115, Accounting for Certain Investments in Debt and Equity Securities. Management determines the appropriate classification of securities at the time of purchase. To date, all marketable securities have been classified as available-for-sale, and are carried at fair value as determined based on quoted market prices with unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) as a component of stockholders’ equity (deficit). The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company has classified all investments as short term.
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities, if any, are included in interest income and expense and have not been material. Realized gains and losses are computed on a specific identification basis. Interest and dividends are included in interest income.
Property and Equipment
Property and equipment, including leasehold improvements, are stated at cost, less accumulated depreciation and amortization. Property and equipment is depreciated using the straight-line method over the estimated useful lives of the assets, which range from three to five years. Leasehold improvements are amortized over the shorter of their estimated useful lives or the related lease term, generally five years. Deposits on equipment consist of amounts paid to vendors as down-payments on certain specialty manufacturing equipment and are not depreciated until the equipment is placed into service.
F-9
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Impairment of Long-Lived Assets
SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, requires losses from impairment of long-lived assets used in operations to be recorded when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. The Company periodically evaluates the carrying value of long-lived assets to be held and used when events and circumstances indicate that the carrying amount of an asset may not be recovered.
Deferred Rent
Lease incentives, including rent holidays and rent escalation provisions, are accrued as deferred rent. The Company recognizes rent expense on a straight-line basis over the term of the lease.
Revenue Recognition
Revenue consists primarily of amounts earned under research grants with the National Institutes of Health (NIH). The Company’s federal government research grants provide for the reimbursement of qualified expenses for research and development as defined under the terms of each grant. Revenue under grants is recognized when earned, and revenue is considered earned as the related qualified research and development expenses are incurred, up to the limit of the approved funding amounts. Because the Company acts as a principal, not as an agent, in research studies performed for the NIH, amounts earned are recorded as revenue. Deferred revenue at December 31, 2006 and March 31, 2007 relates to future services to be performed under a contract with the NIH.
Research and Development
All research and development costs, including those funded by third parties, are expensed as incurred. Research and development costs consist of engineering, product development, test-part manufacturing, testing, developing and validating the manufacturing process, and regulatory related costs. Research and development expenses also include employee compensation, employee and non-employee stock-based compensation, supplies and materials, consultant services, and travel and facilities expenses related to research activities.
Income Taxes
The Company utilizes the liability method of accounting for income taxes as required by SFAS No. 109, Accounting for Income Taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Currently, there is no provision for income taxes as the Company has incurred operating losses to date.
Comprehensive Income (Loss)
SFAS No. 130, Reporting Comprehensive Income, requires components of other comprehensive income, including unrealized gains and losses on available-for-sale investments, to be included as part of accumulated other comprehensive income (loss). The Company displays comprehensive loss and its components as part of the statement of convertible preferred stock and stockholders’ equity (deficit). Comprehensive loss consists of net loss and unrealized gains and losses on available-for-sale investments.
F-10
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Loss per Common Share
The computation of basic and diluted net loss per common share is based on the weighted average number of shares outstanding as follows:
| Years Ended December 31, | Three months ended March 31, |
|||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||
| (unaudited) |
||||||||||||||||||||
| Historical: |
||||||||||||||||||||
| Net loss (numerator) |
$ | (1,756,127 | ) | $ | (4,219,088 | ) | $ | (6,163,554 | ) | $ | (1,317,832 | ) | $ | (5,562,725 | ) | |||||
| Weighted average number of shares outstanding (denominator) |
2,247,611 | 2,254,454 | 2,267,464 | 2,265,863 | 2,271,986 | |||||||||||||||
| Basic and diluted loss per common share |
$ | (0.78 | ) | $ | (1.87 | ) | $ | (2.72 | ) | $ | (0.58 | ) | $ | (2.45 | ) | |||||
| Pro forma: |
||||||||||||||||||||
| Weighted average number of shares outstanding (above) |
2,267,464 | 2,271,986 | ||||||||||||||||||
| Pro forma adjustments to reflect assumed weighted-average effect of conversion of preferred stock (unaudited) |
6,841,334 | 7,172,696 | ||||||||||||||||||
| Shares used to compute pro forma basic and diluted net loss per share (unaudited) |
9,108,798 | 9,444,682 | ||||||||||||||||||
| Pro forma basic and diluted net loss per share (unaudited) |
$ | (0.68 | ) | $ | (0.59 | ) | ||||||||||||||
| Securities excluded from net loss calculations as their impact would be antidilutive (assumes a 1-for-3.82 conversion): |
||||||||||||||||||||
| Convertible preferred stock |
4,973,778 | 4,973,778 | 7,172,696 | 7,172,696 | 7,172,696 | |||||||||||||||
| Stock options |
726,338 | 800,338 | 957,818 | 852,982 | 905,198 | |||||||||||||||
| Preferred stock warrants |
680,638 | 680,638 | 995,759 | 995,759 | 995,759 | |||||||||||||||
| Total |
6,380,754 | 6,454,754 | 9,126,273 | 9,021,437 | 9,073,653 | |||||||||||||||
Stock-Based Compensation
Prior to January 1, 2006, the Company accounted for stock-based employee compensation arrangements using the intrinsic value method in accordance with the recognition and measurement provisions of Accounting Principles Board Opinion (“APB”) No. 25, Accounting for Stock Issued to Employees, and related interpretations, including the Financial Accounting Standards Board Interpretation (“FIN”) No. 44, Accounting for Certain Transactions Involving Stock Compensation, an Interpretation of APB Opinion No. 25 as permitted by SFAS No. 123, Accounting for Stock-Based Compensation. In accordance with APB No. 25, stock-based compensation is calculated using the intrinsic value method and represents the difference between the estimated fair value of our common stock and the per share exercise price of the stock option. Based on this method of accounting, our compensation expense under APB No. 25 was zero.
F-11
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2007 and 2006, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Effective January 1, 2006, the Company adopted the provisions of SFAS No. 123R, Share-Based Payments. In March 2005, the Securities and Exchange Commission issued Staff Accounting Bulletin (“SAB”) No. 107 relating to SFAS No. 123R. The Company has applied the provisions of SAB No. 107 in its adoption of SFAS No. 123R. Under SFAS No. 123R, stock-based awards, including stock options, are recorded at fair value as of the grant date and recognized to expense over the employee’s requisite service period (generally the vesting period) which the Company has elected to amortize on a straight-line basis. Because non-cash stock compensation expense is based on awards ultimately expected to vest, the Company reduces stock compensation expense for estimated forfeitures. For the year ended December 31, 2006 and the three months ended March 31, 2007, the Company’s estimate of forfeitures is zero. The pro forma disclosures previously permitted under SFAS No. 123 are no longer an alternative to financial statement recognition and the Company no longer applies the minimum value method and instead calculates the fair value of its employee stock options using an estimated volatility rate. The Company adopted the provisions of SFAS No. 123R using the prospective transition method. Under the prospective transition method, beginning January 1, 2006, compensation cost recognized includes: (a) compensation cost for all share-based payments granted prior to, but not yet vested as of December 31, 2005, based on the intrinsic value in accordance with the provisions of APB No. 25, and (b) compensation cost for all share-based payments granted subsequent to December 31, 2005, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123R. All awards granted, modified, or settled after the date of adoption are accounted for using the measurement, recognition, and attribution provisions of SFAS No. 123R.
As a result of adopting SFAS No. 123R on January 1, 2006, the net loss for the year ended December 31, 2006 and three months ended March 31, 2006 and 2007 was higher by approximately $38,857, $5,109 and $27,525, respectively, than if the Company had continued to account for stock-based compensation under APB No. 25. As of December 31, 2006 and March 31, 2007, total compensation related to nonvested options not yet recognized in the financial statements was approximately $377,000 and $351,000, respectively, and the weighted average period over which it is expected to be recognized is approximately 3.66 and 3.45 years, respectively. The Company recorded no tax benefit related to these options during the year ended December 31, 2006 or the three months ended March 31, 2006 and 2007, since the Company currently maintains a full valuation allowance offsetting its deferred tax assets.
The Company accounts for equity instruments issued to nonemployees in accordance with the provisions of Emerging Issues Task Force (“EITF”) No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, using a fair-value approach. The equity instruments, consisting of stock options and warrants granted to consultants, are valued using the Black-Scholes valuation model. The measurement of stock-based compensation is subject to periodic adjustments as the underlying equity instruments vest and are recognized as an expense over the period in which services are received.
F-12
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
2. Marketable Securities
The Company invests in highly liquid investment grade securities. The following is a summary of the Company’s marketable securities at December 31, 2005, 2006 and March 31, 2007 (unaudited):
| December 31, 2005 | |||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Market Value | ||||||||||
| Corporate bonds |
$ | 1,440,635 | $ | — | $ | (7,149 | ) | $ | 1,433,486 | ||||
| Euro dollar bonds |
1,204,311 | — | (2,535 | ) | 1,201,776 | ||||||||
| Taxable auction securities |
800,000 | — | — | 800,000 | |||||||||
| Federal agency issue |
1,500,000 | — | (11,521 | ) | 1,488,479 | ||||||||
| Other |
3,510 | — | — | 3,510 | |||||||||
| Total |
$ | 4,948,456 | $ | — | $ | (21,205 | ) | $ | 4,927,251 | ||||
| December 31, 2006 | |||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Market Value | ||||||||||
| Taxable auction securities |
$ | 11,780,000 | $ | — | $ | — | $ | 11,780,000 | |||||
| March 31, 2007 | |||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Market Value | ||||||||||
| Taxable auction securities |
$ | 8,280,000 | $ | — | $ | — | $ | 8,280,000 | |||||
The estimated fair market value amounts have been determined by the Company using available market information. Unrealized gains and losses on marketable securities were reported as a component of accumulated other comprehensive income (loss) in stockholders’ equity (deficit).
As of December 31, 2005 and 2006 and March 31, 2007, the contractual maturities of marketable securities were less than one year.
F-13
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
3. Property and Equipment
Property and equipment consists of the following:
| December 31, | March 31, 2007 |
|||||||||||
| 2005 | 2006 | |||||||||||
| (unaudited) | ||||||||||||
| Manufacturing and lab equipment |
$ | 1,395,251 | $ | 2,893,427 | $ | 3,596,766 | ||||||
| Deposits on equipment |
85,952 | 1,003,808 | 973,403 | |||||||||
| Software |
21,574 | 186,360 | 190,030 | |||||||||
| Leasehold improvements |
26,161 | 566,635 | 600,489 | |||||||||
| Furniture and equipment |
42,026 | 152,864 | 157,857 | |||||||||
| 1,570,964 | 4,803,094 | 5,518,545 | ||||||||||
| Less accumulated depreciation and amortization |
(200,686 | ) | (746,004 | ) | (983,555 | ) | ||||||
| $ | 1,370,278 | $ | 4,057,090 | $ | 4,534,990 | |||||||
4. Deferred Offering Costs
The deferred offering costs as of December 31, 2005 were offset against the proceeds received on the sale of the Series C convertible preferred stock which occurred in February 2006. The deferred offering costs included as of December 31, 2006 will be offset against the proceeds from an anticipated initial public offering of the Company’s common stock. Deferred offering costs as of March 31, 2007 also included costs related to the sale of Series D convertible preferred stock which occurred in April, 2007.
5. Accrued Liabilities
Accrued liabilities consist of the following:
| December 31, | March 31, 2007 | ||||||||
| 2005 | 2006 | ||||||||
| (unaudited) | |||||||||
| Accrued compensation |
$ | 88,623 | $ | 106,044 | $ | 55,954 | |||
| Accrued professional fees |
62,000 | 36,000 | 36,000 | ||||||
| Other |
25,000 | 924 | — | ||||||
| $ | 175,623 | $ | 142,968 | $ | 91,954 | ||||
6. Long Term Debt
In May 2006, the Company entered into an equipment financing arrangement which allowed for advances to the Company for equipment purchased during 2006. These amounts are collateralized by certain of the Company’s qualifying manufacturing and lab equipment and $750,000 which is invested in an interest bearing escrow account and is reflected on the accompanying balance sheet as restricted cash. In the event the balance of the Company’s cash and marketable securities becomes equal to or less than the then remaining balance of the loan at any time during the lease term, the bank has the right to exercise a contingent pledge with respect to all remaining cash and marketable securities.
F-14
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
As of December 31, 2006, $1,621,898 had been advanced under this arrangement. In January 2007, this amount was refinanced into long-term debt with a fixed interest rate of 9.09% and with a 42-month term. The debt has been classified on the accompanying financial statements in accordance with this term loan.
Payments due for long-term debt as of March 31, 2007 are as follows:
| Year ended December 31, |
Long-term Debt | ||
| 2007 (remainder of year) |
$ | 310,381 | |
| 2008 |
448,035 | ||
| 2009 |
490,502 | ||
| 2010 |
307,323 | ||
| $ | 1,556,241 | ||
7. Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Common Stock
As of December 31, 2005 and 2006 and March 31, 2007, the Company was authorized to issue 60,000,000 shares of common stock. As of December 31, 2005, the Company had 2,265,861 shares outstanding and as of December 31, 2006 and March 31, 2007 the Company had 2,271,986 shares outstanding.
The Company had reserved shares of common stock for future issuances as follows (assumes a 1-for-3.82 conversion):
| December 31, | March 31, 2007 | |||||
| 2005 | 2006 | |||||
| (unaudited) | ||||||
| Convertible preferred stock |
||||||
| Shares outstanding |
4,973,778 | 7,172,696 | 7,172,696 | |||
| Shares authorized, but unissued |
5,497,426 | 3,298,508 | 3,298,508 | |||
| Warrants |
||||||
| Series A convertible preferred stock |
549,745 | 549,745 | 549,745 | |||
| Series B convertible preferred stock |
130,893 | 130,893 | 130,893 | |||
| Series C convertible preferred stock |
— | 315,121 | 315,121 | |||
| 2003 Stock Plan |
||||||
| Options outstanding |
800,338 | 957,818 | 905,198 | |||
| Shares available for grant |
204,423 | 40,817 | 224,327 | |||
| 12,156,603 | 12,465,598 | 12,596,488 | ||||
Convertible Preferred Stock
At December 31, 2005, 2006 and March 31, 2007, the Company was authorized to issue 40,000,000 shares of convertible preferred stock. In November 2003 and January 2004, the Company sold a total of 14,000,058 shares of Series A convertible preferred stock for gross proceeds of $8,400,000 and net proceeds of $7,368,751. In November 2004, the Company sold 5,000,000 shares of Series B convertible preferred stock for gross
F-15
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
proceeds of $6,000,000 and net proceeds of $5,357,255. In February 2006, the Company sold 8,400,000 shares of Series C convertible preferred stock for gross proceeds of $16,800,000 and net proceeds of $15,165,512. In addition to receiving fees and convertible preferred stock warrants discussed below, the placement agent has the right to receive through February 2009, up to $2.5 million in the event of a future acquisition of the Company prior to an initial public offering, resulting in a change in control, as defined in the agreement.
At December 31, 2005, convertible preferred stock consisted of the following:
| Series |
Designated Shares |
Shares Issued and Outstanding |
Aggregate Liquidation Preference | ||||
| Series A |
16,150,000 | 14,000,058 | $ | 8,400,035 | |||
| Series B |
6,000,000 | 5,000,000 | 6,000,000 | ||||
| Total |
22,150,000 | 19,000,058 | $ | 14,400,035 | |||
At December 31, 2006 and March 31, 2007, convertible preferred stock consisted of the following:
| Series |
Designated Shares |
Shares Issued and Outstanding |
Aggregate Liquidation Preference | ||||
| Series A |
16,150,000 | 14,000,058 | $ | 8,400,035 | |||
| Series B |
6,000,000 | 5,000,000 | 6,000,000 | ||||
| Series C |
9,700,000 | 8,400,000 | 16,800,000 | ||||
| Total |
31,850,000 | 27,400,058 | $ | 31,200,035 | |||
In April 2007, the Company issued 4,456,500 shares of Series D convertible preferred stock at $3.00 per share for net proceeds of approximately $12,400,000. Dividends, liquidation preferences, conversion and voting rights of Series D convertible preferred stock are consistent with those of Series A, B and C convertible preferred stock. In conjunction with this offering, the placement agent received warrants to purchase 253,290 shares of Series D convertible preferred stock which were granted at an exercise price of $3.30 per share, and are fully exercisable at the earlier of one year from issuance or the completion of an initial public offering of the Company’s common stock, expire after seven years and have an estimated fair value of $450,000. In connection with the Series D convertible preferred stock offering, the Company paid the placement agent $758,870 as commission and $100,000 for expenses.
The rights and preferences of the convertible preferred stock are as follows:
Dividends
The convertible preferred stock shall be entitled to receive noncumulative dividends in preference to any dividend on common stock payable only if declared by the Board of Directors. As of December 31, 2005 and 2006 and March 31, 2007, the Board of Directors had not declared any dividends.
Liquidation Preference
In the event of any liquidation or winding up of the Company, including in the event of the merger, consolidation and sale of the Company, the holders of preferred stock shall be entitled to receive, in preference to
F-16
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
the holders of the common stock, a per share amount equal to the original purchase price for such shares, subject to appropriate adjustment, plus all declared but unpaid dividends. After the payment of the liquidation preference to the holders of the preferred stock, the remaining assets shall be distributed ratably to the holders of the common stock.
A sale, merger, reorganization, liquidation, dissolution or winding up of the Company may, in certain circumstances, be deemed to be a liquidation and trigger the liquidation preferences associated with the outstanding shares of convertible preferred stock. Because a change in control could occur and not be solely within the control of the Company, all convertible preferred stock has been deemed to be redeemable and classified outside of permanent equity in the accompanying consolidated balance sheets, as required by EITF Topic D-98, Classification and Measurement of Redeemable Securities. However, because the timing of any such redemption is uncertain, the Company will not accrete the carrying value of the convertible preferred stock to its liquidation preference value until it becomes probable that redemption will occur.
Conversion
The holders of the convertible preferred stock shall have the right to convert the shares of preferred stock held by such holders, at any time, into shares of common stock on a 1-for-3.82 basis, subject to adjustment for anti-dilution. Upon conversion, any declared but unpaid dividends on the preferred stock will be paid in additional shares of common stock.
The convertible preferred stock shall be automatically converted into common stock, at the then applicable conversion ratio, upon the closing of a public offering of shares of common stock at a per share price not less than the then applicable conversion price (as adjusted for stock splits, stock dividends, recapitalizations, etc.).
Voting Rights
The preferred stock will vote together with the common stock, and not as separate classes, except as specifically provided below or as otherwise required by law. Each share of preferred stock shall have a number of votes equal to the number of shares of common stock.
Unless an affirmative vote of 50 percent of the combined outstanding shares of preferred stock, voting separately as a class, is obtained, the Company shall not undertake any of the following: (i) declaration or payment of any dividend or other distribution or payment on the (or the redemption, purchase or other acquisition for value of any) capital stock of the Company or any subsidiary; (ii) any liquidation, dissolution, recapitalization or reorganization of the Company; (iii) transfer or disposition of assets or rights with a value of more than $1,000,000; and/or (iv) any amendment to the Company’s certificate of incorporation that changes or alters any of the preferences, voting powers or other rights and privileges of preferred stock.
Registration Rights
The preferred shareholders and warrant holders were granted registration rights that provide these holders the right to request, 180 days after the completion of a qualifying initial public offering, that the Company file a registration statement to register under the Securities Act the common stock that would be issued upon conversion of the preferred shares or exercise of the warrants. Thereupon the Company is obligated to use commercially reasonable efforts to file a timely registration statement. These registration rights are subject to
F-17
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
certain conditions and limitations, including our right, based on advise of the lead managing underwriter of a future offering, to limit the number of shares included in any such registration under certain circumstances.
Preferred Stock Warrant Liability
In connection with the convertible preferred stock offerings referred to above, the placement agent received warrants to purchase convertible preferred stock. These warrants are fully exercisable after one year from issuance and expire after seven years. The exercise price of these warrants is equal to 110% of the offering price of the underlying convertible preferred stock. On the closing of an initial public offering, these warrants will convert into warrants to purchase shares of common stock at the then applicable conversion rate for the related preferred stock (currently 1-for-3.82). The grant dates, number of warrants, exercise price and estimated fair value of the warrants are as noted below.
| Estimated Fair Value | ||||||||||||||||
| Series of convertible preferred |
Grant Date | Number of Shares |
Exercise Price |
December 31, | March 31, | |||||||||||
| 2005 | 2006 | 2007 | ||||||||||||||
| (unaudited) | ||||||||||||||||
| Series A |
1/30/2004 | 2,100,000 | $ | 0.66 | $ | 1,134,000 | $ | 1,470,000 | $ | 3,591,000 | ||||||
| Series B |
11/01/2004 | 500,000 | 1.32 | 270,000 | 335,000 | 800,000 | ||||||||||
| Series C |
2/24/2006 | 1,203,750 | 2.20 | — | 818,550 | 1,913,963 | ||||||||||
| 3,803,750 | $ | 1,404,000 | $ | 2,623,550 | $ | 6,304,963 | ||||||||||
The Company has accounted for these warrants under the provisions of Financial Accounting Standards Board Staff Position (“FSP”) No. 150-5, Issuer’s Accounting under Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that Are Redeemable, an interpretation of SFAS No. 150, Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity. Pursuant to FSP No. 150-5, freestanding warrants for shares that are either puttable or warrants for shares that are redeemable are classified as liabilities on the balance sheet at fair value. In connection with the grant of the warrants to purchase Series A and Series B convertible preferred stock in 2004 and Series C convertible preferred stock in 2006, the Company recorded the initial fair values of the warrants of $413,080, $159,831 and $928,625, respectively, as a preferred stock warrant liability. At the end of each reporting period, changes in fair value during the period are recorded as a component of other income or expense.
The fair value of the above warrants was determined using the Black-Scholes valuation model using the following assumptions:
| Year ended December 31, | Three months ended March 31, | |||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||
| (unaudited) | ||||||||||
| Weighted-average risk-free interest rate |
3.85% | 4.36% | 4.70% | 4.70% |
4.55% | |||||
| Weighted-average expected life (in years) |
6.24 | 5.24 | 4.85 | 4.85 |
4.60 | |||||
| Expected dividend yield |
0% | 0% | 0% | 0% |
0% | |||||
| Weighted-average expected volatility |
108% | 94% | 75% | 75% |
70% | |||||
For the years ended December 31, 2004, 2005 and 2006, and for the three month period ended March 31, 2006 and 2007, the Company recorded $254,089, $577,000, $290,925, $72,731 and $3,681,413, respectively, as
F-18
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2007 and 2006, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
other expense for the increase in fair value of all preferred stock warrants. The Company will continue to adjust the liabilities for changes in fair value until the earlier of the exercise of the warrants to purchase shares of convertible preferred stock or the completion of a liquidation event, including the completion of an initial public offering, at which time the liabilities will be reclassified to stockholders’ equity (deficit) when the warrants are converted to common stock warrants.
Stock Option Plan
Under the Company’s 2003 Stock Option Plan, the Company’s Board of Directors has authorized the grant of options to employees and nonemployees for the issuance of up to 1,047,121 shares of the Company’s common stock. In March 2007, the Board of Directors reserved an additional 130,890 shares to be issued under the 2003 Stock Option Plan. All options granted have a term of ten years from the date of the grant and generally become fully exercisable within four years of continued employment or service at a rate defined in each option agreement.
A summary of the Company’s stock option activity and related information is as follows:
| Options Outstanding | |||||||||
| Shares Available for Grant |
Number of Options |
Weighted Average Exercise Price | |||||||
| Balance at inception (December 10, 1996) |
— | — | $ | — | |||||
| Shares authorized |
1,047,121 | — | $ | — | |||||
| Options granted |
(565,451 | ) | 565,451 | $ | 0.48 | ||||
| Balance at December 31, 2003 |
481,670 | 565,451 | $ | 0.48 | |||||
| Granted |
(203,483 | ) | 203,483 | $ | 1.43 | ||||
| Exercised |
— | (24,925 | ) | $ | 0.96 | ||||
| Cancelled |
17,671 | (17,671 | ) | $ | 0.59 | ||||
| Balance at December 31, 2004 |
295,858 | 726,338 | $ | 0.72 | |||||
| Granted |
(96,999 | ) | 96,999 | $ | 2.64 | ||||
| Exercised |
— | (17,435 | ) | $ | 0.96 | ||||
| Cancelled |
5,564 | (5,564 | ) | $ | 1.03 | ||||
| Balance at December 31, 2005 |
204,423 | 800,338 | $ | 0.95 | |||||
| Granted |
(196,477 | ) | 196,477 | $ | 3.82 | ||||
| Exercised |
— | (6,126 | ) | $ | 0.59 | ||||
| Cancelled |
32,871 | (32,871 | ) | $ | 1.89 | ||||
| Balance at December 31, 2006 |
40,817 | 957,818 | $ | 1.51 | |||||
| Granted (unaudited) |
(17,016 | ) | 17,016 | $ | 3.82 | ||||
| Cancelled (unaudited) |
69,636 | (69,636 | ) | $ | 1.90 | ||||
| Increase in authorized shares |
130,890 | — | $ | — | |||||
| Balance at March 31, 2007 (unaudited) |
224,327 | 905,198 | $ | 1.52 | |||||
There were options to purchase 460,266, 625,828 and 645,745 shares of common stock that were exercisable at December 31, 2005, 2006 and March 31, 2007 respectively, at a weighted-average exercise price of $0.55, $0.70 and $0.84, respectively.
F-19
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Information about outstanding stock options is as follows:
| December 31, 2006 | March 31, 2007 (unaudited) | |||||||||||
| Exercise Price Per Share |
Options Outstanding |
Weighted Average Remaining Contractual Life (in years) |
Options Exercisable |
Options Outstanding |
Weighted Average Remaining Contractual Life (in years) |
Options Exercisable | ||||||
| $0.38 |
250,005 | 6.72 | 243,662 | 250,005 | 6.47 | 244,649 | ||||||
| $0.42 |
209,425 | 6.72 | 209,425 | 209,425 | 6.47 | 209,425 | ||||||
| $0.96 |
156,363 | 7.21 | 110,474 | 123,640 | 6.91 | 101,755 | ||||||
| $2.29 |
128,006 | 8.19 | 57,716 | 101,827 | 7.96 | 52,132 | ||||||
| $3.82 |
214,019 | 9.64 | 4,551 | 220,301 | 9.44 | 37,784 | ||||||
| $0.38-$3.82 |
957,818 | 7.65 | 625,828 | 905,198 | 7.42 | 645,745 | ||||||
The weighted-average grant date fair value of the options granted during the years ended December 31, 2004, 2005 and 2006 was $0.31, $0.61 and $2.98 per share, respectively. The weighted-average grant date fair value of the options granted during the three months ended March 31, 2006 and 2007, was $3.13 and $2.79, respectively.
Stock Options under SFAS 123R
On January 1, 2006, the Company adopted the provisions of SFAS No. 123R, Share-Based Payment. SFAS No. 123R establishes accounting for stock-based awards made to employees and directors. Accordingly, stock-based compensation expense is measured at grant date, based on the fair value of the award, and is recognized as expense over the remaining requisite service period. Total employee stock-based compensation of $38,857, $5,109 and $27,525, respectively was recorded during the year ended December 31, 2006 and three months ended March 31, 2006 and 2007.
During 2006 and the three months ended March 31, 2007, the Company issued options to employees and directors with exercise prices that, at the time of grant, the board of directors determined to approximate the fair value of the Company’s common stock, taking into consideration a number of factors including the issuance price of shares of the Company’s convertible preferred shares, the preferential terms and conditions of the convertible preferred stock, the status of scientific research and development efforts and associated milestones and the likelihood of achieving a liquidity event for the share of the Company’s common stock. The board of directors has reassessed the fair value of these grants given the anticipation of completing an initial public offering and the timing of key events that occurred during 2006 and the three months ended March 31, 2007. Reassessed value was determined using valuations performed by an independent valuation consultant (as discussed below) that used a combination of income and market valuation approaches. For dates not coinciding with the valuations, reassessed value was determined by evaluating the impact of milestones achieved during those periods and general progress in the business, as well as the proximity of the grant date to the date of the corresponding valuation.
F-20
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Information on employee and director options granted in 2006 and the first three months of 2007 is as follows:
| Grant Date | Number of Shares Granted |
Exercise Price per Share |
Reassessed Fair Value Per Share |
Intrinsic Value | |||||||
| 2/12/2006 | 50,589 | $ | 3.82 | $ | 3.82 | $ | — | ||||
| 12/11/2006 | 97,449 | 3.82 | 3.82 | — | |||||||
| 1/5/2007 | 17,016 | 3.82 | 4.05 | 0.23 | |||||||
The fair value of the underlying common stock for these options was assessed by the Company based on retrospective valuations performed by LECG, LLC, or LECG, an independent valuation consultant, as of December 31, 2005 and December 31, 2006 and a contemporaneous valuation performed by LECG on October 31, 2006.
The fair value of each employee option grant in the year ended December 31, 2006 and the three months ended March 31, 2006 and 2007 was estimated on the date of grant using the Black-Scholes valuation model with the following assumptions.
| Year
ended |
Three months ended March 31, | ||||||||
| 2006 | 2007 | ||||||||
| (unaudited) | |||||||||
| Weighted average risk-free interest rate |
4.53% |
|
4.58% |
4.67% | |||||
| Weighted-average expected life (in years) |
6.25 |
|
6.25 |
5 | |||||
| Expected dividend yield |
0% |
|
0% |
0% | |||||
| Weighted average expected volatility |
91% |
|
101% |
81% | |||||
| Weighted-average estimated fair value of employee options |
$ | 2.98 | $ |
3.13 |
$ | 2.79 | |||
The Company’s computation of expected volatility for the year ended December 31, 2006 and the three months ended March 31, 2006 and 2007 is based on an average of the historical volatility of a peer-group of similar companies. The Company’s computation of expected term utilizes the simplified method in accordance with SAB No. 107. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The Company recognizes stock-based compensation expense for the fair values of these awards on a straight-line basis over the requisite service period of each of these awards.
As of December 31, 2006, the weighted-average remaining contractual term for outstanding stock options and for exercisable stock options was 7.7 years and 7.0 years, respectively, and the intrinsic value of these options was approximately $2,435,000 and $2,098,000, respectively. The aggregate intrinsic value represents the total pre-tax intrinsic value, based on the Company’s estimated stock price of $4.05 per share as of December 31, 2006, which would have been received by the option holders had all option holders exercised their options on December 31, 2006. This amount changes based on the estimated value of the Company’s common stock. Total intrinsic value of options exercised for the year ended December 31, 2006 was $19,800, based on 6,126 shares exercised, an estimated stock price during 2006 of $3.82 per share, and an average exercise price of $0.59 per share exercised. During the year ended December 31, 2006, the Company granted 196,477 stock options to employees and directors with an estimated total grant-date fair value of $2.98 per share.
F-21
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
Cash received from option exercises for the years ended December 31, 2004, 2005 and 2006 was $23,782, $16,650 and $3,600, respectively. The Company recorded no tax benefit related to options exercised during 2004, 2005 and 2006.
Stock Options Granted to Nonemployees
The Company granted 18,326, 21,600, and 48,439 options to consultants for services in the years ended December 31, 2004, 2005, and 2006, respectively. No options were granted to consultants in the three months ended March 31, 2007. The exercise price of the consultant stock options ranges from $0.96 to $3.82 per share. Information on nonemployee options granted in 2006 and the first three months of 2007 is as follows:
| Grant Date | Number of Shares Granted |
Exercise Price per Share |
Reassessed Fair Value Per Share |
Intrinsic Value | |||||||
| 2/12/2006 | 9,949 | $ | 3.82 | $ | 3.82 | $ | — | ||||
| 12/11/2006 | 38,490 | 3.82 | 3.82 | — | |||||||
The fair value of the underlying common stock for these options was assessed by the Company based on retrospective valuations performed as of December 31, 2005 and December 31, 2006 and a contemporaneous valuation performed on October 31, 2006.
The following table shows the assumptions used to compute the stock-based compensation expense for stock options granted to nonemployees during the years ended December 31, 2004, 2005 and 2006 and the three months ended March 31, 2006 and 2007, using the Black Scholes valuation model.
| Year ended December 31, | Three months ended March 31, | |||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||
| (unaudited) | ||||||||||
| Weighted-average risk-free interest rate |
3.83% | 3.94% | 4.13% | 4.00% |
4.36% | |||||
| Weighted-average contractual life (in years) |
9.14 | 8.31 | 8.21 | 8.31 |
8.01 | |||||
| Expected dividend yield |
0% | 0% | 0% | 0% |
0% | |||||
| Weighted-average expected volatility |
101% | 101% | 86% | 101% |
83% | |||||
The estimated fair value of options granted to consultants which vested during the years ended December 31, 2004, 2005, and 2006 and the three months ended March 31, 2006 and 2007 was $18,391, $44,266, $92,313, $22,617 and $49,402, respectively, and was charged to research and development expense.
8. Income Taxes
The Company originally elected to be treated under the Internal Revenue Code as a subchapter S corporation, commensurate with its inception on December 10, 1996. Accordingly, the Company did not pay federal corporate income taxes on its taxable income nor was it allowed a net operating loss carryover or carryback as a deduction. Instead, the stockholders were responsible for individual federal income taxes on their respective shares of the Company’s taxable income or loss. The Company’s subchapter S corporation status was terminated effective November 5, 2003 due to the capitalization restructuring to include a second class of stock. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
F-22
In July 2006, the FASB issued Interpretation No. 48, Accounting for Uncertainty in Income Taxes, or FIN 48. FIN 48 is an interpretation of FASB Statement No. 109, Accounting for Income Taxes. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of measurement and recognition in accounting for income taxes. In addition, FIN 48 provides guidance on derecognition, classification, interest and penalties, and accounting in interim periods and requires expanded disclosure with respect to the uncertainty in income taxes. The Company became subject to the provisions of FIN 48 as of January 1, 2007. The Company believes that its income tax filing positions and deductions will be sustained on audit and does not anticipate any adjustments that will result in a material change to its financial position. Therefore, no reserves for uncertain income tax positions have been recorded pursuant to FIN 48. In addition, the Company did not record a cumulative effect adjustment related to the adoption of FIN 48.
The Company’s policy for recording interest and penalties associated with audits is to record such items as a component of income before taxes. Penalties and interest paid or received are recorded in interest expense or interest income, respectively. During the three months ended March 31, 2007, the Company did not record any interest income, interest expense, or penalties related to the settlement of audits for prior periods. Tax years 2003 through 2006 are subject to examination by the United States Federal tax authorities.
At December 31, 2006, the Company had net operating loss carryforwards for federal and state income tax purposes of approximately $10,600,000. The federal and state net operating loss carryforwards will expire from 2023 to 2026. Additionally, at December 31, 2006 the Company had federal and state research and development tax credits of $137,000 which expire from 2023 to 2026.
In accordance with Section 382 of the Internal Revenue Code, a change in ownership of greater than 50% within a three-year period will place an annual limitation on the Company’s ability to utilize its existing net operating loss carryforwards. The Company may be subject to these annual limitations and, therefore, unable to fully utilize the net operating loss carryforwards.
Significant components of the Company’s deferred tax assets and liabilities approximated the following:
| December 31, | ||||||||
| 2005 | 2006 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 1,801,000 | $ | 4,043,000 | ||||
| R&D credits |
137,000 | 137,000 | ||||||
| Other |
147,000 |
|
224,000 |
| ||||
| Total deferred tax assets |
2,085,000 |
|
4,404,000 |
| ||||
| Deferred tax liabilities: |
||||||||
| Depreciation |
(27,000 | ) | (143,000 | ) | ||||
| Total deferred tax liabilities |
(27,000 | ) | (143,000 | ) | ||||
| Less valuation allowance |
(2,058,000 | ) | (4,261,000 | ) | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
The Company has recognized a valuation allowance equal to the net deferred tax assets due to management’s assessment that it is not more likely than not that such deferred tax assets will be realized. The tax valuation allowance increased by $437,000, $1,618,000 and $2,203,000 for the years ended December 31, 2004, 2005 and 2006, respectively.
F-23
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
9. Commitments
The Company currently leases laboratory, manufacturing and office space and equipment under noncancelable operating leases which sometimes provide for rent holidays and escalating payments. Rent under operating leases is recognized on a straight-line basis beginning with lease commencement through the end of the lease term. For the years ended December 31, 2004, 2005 and 2006, and for the three months ended March 31, 2006 and 2007, rental expense was approximately $112,000, $212,000, $471,000, $57,000 and $146,000, respectively. Future minimum lease payments under all noncancelable operating leases at March 31, 2007 are as follows:
| Year ending December 31, |
|||
| 2007 (remainder of year) |
$ | 339,875 | |
| 2008 |
463,392 | ||
| 2009 |
400,991 | ||
| 2010 |
263,154 | ||
| 2011 |
91,605 | ||
| Total minimum lease payments |
$ | 1,559,017 | |
The Company has entered into consulting and development agreements with some of its advisors, including some surgeon advisors. The Company has agreed to pay some of the surgeon advisors a portion of the net after-tax profits attributable to the sale of specific spine, hip and knee implant product candidates for which the surgeon advisors provided the Company with consulting and related services related to the conceptualization, development, testing, clearance, approval and/or related matters involving implant product candidates. Because more than one of these surgeon advisors contribute to development efforts, the Company is obligated to pay royalties to as many as five surgeon advisors in connection with some of its product candidates. These agreements shall continue until the later of (a) ten years from the date of the agreements, and (b) the expiration of the patent rights relating to the devices covered by the agreements, when rights have been assigned by the individuals to the Company.
In December 2006, the Company entered into an agreement to license patent rights directed to a manufacturing process for porous ceramic for use in the Company’s product candidates that will incorporate its CSC technology. At the time this agreement was signed, the Company paid $50,000. During February 2007, the Company paid an additional $100,000 upon the transfer of the technology to the Company. The Company is obligated to pay an additional $100,000 upon FDA clearance of the first product in the United States which utilizes the licensed technology. The Company is also obligated to pay future royalties on net sales of products which utilize this technology.
The Company has executed agreements with certain executive officers of the Company which, upon the occurrence of certain events related to a change of control, call for payments to the executives equal to two to three times annual salary and accelerated vesting of previously granted stock options.
10. Related Party Transactions
One of the Company’s co-founders and a member of its board of directors is also the sole member and president of Joint Enterprises, L.C., a Utah limited liability company. The Company and Joint Enterprises, L.C. previously entered into an Assignment Agreement, dated August 1, 2001, which has been amended as of
F-24
AMEDICA CORPORATION
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of March 31, 2007, for the three months ended March 31, 2006 and 2007, and for the period from December 10, 1996 (inception) to March 31, 2007 is unaudited)
August 12, 2005. Pursuant to this agreement, the Company acquired rights to the PreVent Cement Restrictor in exchange for its agreement to pay a one-time payment of $25,000 and to pay royalties equal to $2.50 per unit. Joint Enterprises, L.C. also has the option to elect to receive nonqualified stock options to purchase shares of the Company’s common stock in lieu of cash payments, subject to approval by the Company’s board of directors. The Company made the $25,000 payment to this director in September 2004. As of the date hereof, no units of this product have been sold and no royalties for this product have been paid pursuant to this agreement.
The Company completed offerings of its shares of Series A, Series B, Series C and Series D convertible preferred stock through Creation Capital LLC, its placement agent for each of these offerings. The Chief Executive Officer of Creation Capital joined the Company’s Board of Directors in December 2006. In connection with the closing of the Series A convertible preferred stock offering, the Company paid its placement agent $611,700 as commission and $75,000 for expenses in 2003 and $182,640 as commission and $20,875 for expenses in 2004. In connection with the Series B convertible preferred stock offering, the Company paid its placement agent $480,000 as commission and $40,000 for expenses in 2004. In connection with the Series C convertible preferred stock offering, the Company paid its placement agent $1,511,265 as commission and $75,000 for expenses in 2006. Through February 2009, the placement agent also has the right to receive up to $2.5 million in the event of a future acquisition of the capital stock of the Company prior to an initial public offering, resulting in a change in control.
11. 401(k) Plan
Effective June 1, 2004, the Company adopted a defined contribution retirement plan under Section 401(k) of the Internal Revenue Code. The plan covers substantially all employees. Eligible employees may contribute amounts to the plan, via payroll withholdings, subject to certain limitations. The plan permits, but does not require, additional matching contributions to the plan by the Company on behalf of the participants in the plan. To date, the Company has not made any matching contributions to the plan.
12. Reverse Stock Split
The Company effected a one-for-3.82 reverse stock split of the Company’s common stock on July 26, 2007. All common share and per share amounts have been retroactively adjusted to reflect the reverse stock split.
F-25

PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth an itemization of the various costs and expenses, all of which we will pay, in connection with the issuance and distribution of the securities being registered. All of the amounts shown are estimated except the SEC Registration Fee, The NASDAQ Global Market Listing Fee and the NASD Filing Fee.
| SEC Registration Fee |
$ | 2,463 | |
| The NASDAQ Global Market Listing Fee |
|
100,000 | |
| NASD Filing Fee |
8,522 | ||
| Printing and Engraving Fees |
200,000 | ||
| Legal Fees and Expenses |
2,000,000 | ||
| Accounting Fees and Expenses |
400,000 | ||
| Blue Sky Fees and Expenses |
15,000 | ||
| Transfer Agent and Registrar Fees |
5,000 | ||
| Miscellaneous |
19,015 | ||
| Total |
$ | 2,750,000 | |
Item 14. Indemnification of Directors and Officers.
Our amended and restated certificate of incorporation and amended and restated bylaws provide that each person who was or is made a party or is threatened to be made a party to or is otherwise involved (including, without limitation, as a witness) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he or she is or was one of our directors or officers or is or was serving at our request as a director, officer, employee or agent of another corporation, or of a partnership, joint venture, trust or other enterprise, including service with respect to an employee benefit plan, whether the basis of such proceeding is alleged action in an official capacity as a director, officer or trustee or in any other capacity while serving as a director, officer or trustee, shall be indemnified and held harmless by us to the fullest extent authorized by the Delaware General Corporation Law against all expense, liability and loss (including attorneys’ fees, judgments, fines, ERISA excise taxes or penalties and amounts paid in settlement) reasonably incurred or suffered in connection with legal proceedings. A director or officer will not receive indemnification if he or she is found not to have acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interest.
Section 145 of the Delaware General Corporation Law permits a corporation to indemnify any director or officer of the corporation against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reasonable cause to believe his or her conduct was unlawful. In a derivative action, (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that such person is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
II-1
Pursuant to Section 102(b)(7) of the Delaware General Corporation Law, Article Tenth of our amended and restated certificate of incorporation eliminates the liability of a director to us or our stockholders for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
| • | from any breach of the director’s duty of loyalty to us or our stockholders; |
| • | from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| • | under Section 174 of the Delaware General Corporation Law; or |
| • | from any transaction from which the director derived an improper personal benefit. |
We carry insurance policies insuring our directors and officers against certain liabilities that they may incur in their capacity as directors and officers. In addition, we expect to enter into indemnification agreements with each of our directors and executive officers prior to completion of the offering.
Additionally, reference is made to the Underwriting Agreement filed as Exhibit 1.1 hereto, which provides for indemnification by the underwriters of Amedica Corporation, our directors and officers who sign the registration statement and persons who control Amedica Corporation, under certain circumstances.
Item 15. Recent Sales of Unregistered Securities.
In the three years preceding the filing of this registration statement, we have sold the following securities that were not registered under the Securities Act.
(a) Issuances of Capital Stock and Warrants
In late 2004 through the date hereof, we completed offerings of our Series A, Series B, Series C and Series D convertible preferred stock through Creation Capital LLC, as placement agent. The sale and issuance of the securities set forth below were deemed to be exempt from registration under the Securities Act by virtue of Section 4(2) or Regulation D promulgated thereunder.
| • | Between November 5, 2003 and January 28, 2004, we raised $8.4 million in gross proceeds and sold 14,000,058 shares of our Series A convertible preferred stock at a purchase price of $0.60 per share to 102 accredited investors. In connection with this financing, we also agreed to grant Creation Capital LLC, as partial compensation for its services, warrants to purchase 2,100,000 shares of our Series A convertible preferred stock at a purchase price of $0.66 per share. At the request of Creation Capital LLC, we issued such warrants to 20 designees of Creation Capital LLC, each of whom is an accredited investor. These warrants are currently exercisable through the seventh anniversary of the date of issuance of such warrants. |
| • | Between October 25, 2004 and November 9, 2004, we raised $6.0 million in gross proceeds and sold 5,000,000 shares of our Series B convertible preferred stock at a purchase price of $1.20 per share to 72 accredited investors. In connection with this financing, we also agreed to grant Creation Capital LLC, as partial compensation for its services, warrants to purchase 500,000 shares of our Series B convertible preferred stock at a purchase price of $1.32 per share. At the request of Creation Capital LLC, we issued such warrants to 13 designees of Creation Capital LLC, each of whom is an accredited investor. These warrants are currently exercisable through the seventh anniversary of the date of issuance of such warrants. |
| • | On February 24, 2006, we raised $16.8 million in gross proceeds and sold 8,400,000 shares at $2.00 per share of our Series C convertible preferred stock to 114 accredited investors. In connection with this financing, we also agreed to grant Creation Capital LLC, as partial compensation for its services, warrants to purchase 1,203,750 shares of Series C convertible preferred stock at a purchase price of $2.20 per share. At the request of Creation Capital LLC, we issued such warrants to 19 designees of |
II-2
| Creation Capital LLC, each of whom is an accredited investor. These warrants are currently exercisable through the seventh anniversary of the date of issuance of such warrants. |
| • | On April 17, 2007 and April 27, 2007, we raised $10.2 million and $3.2 million in gross proceeds, respectively, and sold 3,404,000 and 1,052,500 shares, respectively, at $3.00 per share of our Series D convertible preferred stock to 60 accredited investors. In connection with this financing, we also agreed to grant Creation Capital LLC, as partial compensation for its services, warrants to purchase 253,290 shares of Series D convertible preferred stock at a purchase price of $3.30 per share, of which, at the request of Creation Capital LLC, a warrant to purchase 30,000 shares was issued to its designee, an accredited investor. Upon completion of this offering, these warrants will become exercisable through the seventh anniversary of the date of issuance of such warrants. |
(b) Certain Grants and Exercises of Stock Options
The sale and issuance of the securities described below were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving a public offering or transactions pursuant to compensatory benefit plans and contracts relating to compensation as provided under Rule 701.
Pursuant to our 2003 Stock Option Plan and related stock option agreements, we have issued options to purchase an aggregate of 1,208,056 shares of common stock as of June 30, 2007. Of these options:
| • | options to purchase 129,149 shares of common stock have been canceled or lapsed without being exercised; |
| • | options to purchase 106,024 shares of common stock have been exercised; and |
| • | options to purchase a total of 972,888 shares of common stock are currently outstanding, at a weighted average exercise price of $2.55 per share. |
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
| Exhibit Number |
Description of Exhibit | |
| 1.1** | Form of Underwriting Agreement. | |
| 3.1** | Amended and Restated Certificate of Incorporation of Amedica Corporation, as amended. | |
| 3.2** | Certificate of Amendment to Restated Certificate of Incorporation of Amedica Corporation. | |
| 3.3** | Amended and Restated Certificate of Incorporation of Amedica Corporation to be effective upon completion of this offering. | |
| 3.4** | Amended and Restated By-Laws of Amedica Corporation. | |
| 3.5** | Amended and Restated By-Laws of Amedica Corporation to be effective upon completion of this offering. | |
| 4.1** | Form of Common Stock Certificate. | |
| 4.2** | Third Amended and Restated Registration Rights Agreement by and among Amedica Corporation and certain securityholders of Amedica Corporation dated as of May 15, 2007. | |
| 4.3** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of March 1, 2004. | |
| 4.4** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as October 25, 2004. | |
| 4.5** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of February 24, 2006. | |
II-3
| Exhibit Number |
Description of Exhibit | |
| 4.6** | Series D Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of April 27, 2007. | |
| 5.1 | Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., counsel to Amedica Corporation, with respect to the legality of securities being registered. | |
| 10.1** | Form of Subscription Agreement for Series C Convertible Preferred Stock. | |
| 10.2** | Form of Subscription Agreement for Series D Convertible Preferred Stock. | |
| 10.3†** | Amedica Corporation 2003 Stock Option Plan. | |
| 10.4†** | Form of 2003 Incentive Stock Option Agreement. | |
| 10.5†** | Form of 2003 Non-Qualified Stock Option Agreement. | |
| 10.6†** | Employment Offer Letter by and between Amedica Corporation and Eugene B. Jones dated April 2, 2004. | |
| 10.7†** | Employment Offer Letter by and between Amedica Corporation and Bryan J. McEntire dated May 29, 2004. | |
| 10.8†** | Employment Offer Letter by and between Amedica Corporation and Robert M. Wolfarth dated November 11, 2004. | |
| 10.9†** | Employment Offer Letter by and between Amedica Corporation and Cameron G. Rouns dated December 17, 2004. | |
| 10.10†** | Employment Offer Letter by and between Amedica Corporation and Warionex (“Jose”) Belen dated May 31, 2005. | |
| 10.11†** | Employment Offer Letter by and between Amedica Corporation and Reyn E. Gallacher dated January 3, 2006. | |
| 10.12†** | Employment Offer Letter by and between Amedica Corporation and Kenneth W. Ludwig dated May 10, 2006. | |
| 10.13†** | Severance Agreement by and between Amedica Corporation and Ashok C. Khandkar dated May 23, 2005. | |
| 10.14†** | Severance Agreement by and between Amedica Corporation and Bryan J. McEntire dated May 23, 2005. | |
| 10.15†** | Severance Agreement by and between Amedica Corporation and Warionex (“Jose”) Belen dated as of February 14, 2006. | |
| 10.16†** | Severance Agreement by and between Amedica Corporation and Reyn E. Gallacher dated March 27, 2007. | |
| 10.17†** | Severance Agreement by and between Amedica Corporation and Kenneth W. Ludwig dated March 27, 2007. | |
| 10.18†** | Separation Agreement by and between Amedica Corporation and Eugene B. Jones dated January 5, 2007. | |
| 10.19** | Assignment Agreement by and between Joint Enterprises, L.C. and Amedica Corporation dated August 1, 2001. | |
| 10.20** | Amendment to Assignment by and between Joint Enterprises, L.C. and Amedica Corporation dated August 12, 2005. | |
| 10.21** | Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of March 22, 2004. | |
| 10.22** | First Amendment to Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of May 11, 2005. | |
II-4
| Exhibit Number |
Description of Exhibit | |
| 10.23** | Second Amendment to Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of August 15, 2006. | |
| 10.24n** | Letter Agreement between Creation Capital LLC and Amedica Corporation dated November 14, 2005. | |
| 10.25n** | Letter Agreement between Creation Capital LLC and Amedica Corporation dated March 26, 2007. | |
| 10.26n** | Master Lease Agreement by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of April 28, 2006. | |
| 10.26.1** | Amendment of Master Lease by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of May 23, 2007. | |
| 10.27** | Continuing Pledge Agreement by and between Chase Equipment Leasing, Inc. dated as of April 28, 2006. | |
| 10.27.1** | Security Agreement by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of June 30, 2006. | |
| 10.28** | Industrial Building Lease between 560 Arapeen LLC, Seventh Avenue LLC, First Avenue LLC, Alaska Limited Liability Companies and Amedica Corporation dated as of February 20, 2006. | |
| 10.29n** | Product Development and License Agreement by and between Amedica Corporation and Dytech Corporation Ltd. dated December 20, 2006. | |
| 10.30n** | Development Agreement by and between Amedica Corporation and Jeffrey C. Wang, M.D. dated as of May 8, 2004. | |
| 10.31n** | Amendments to Development Agreement by and between Amedica Corporation and Jeffrey C. Wang, M.D. dated as of June 2, 2006. | |
| 10.32n** | Development Agreement by and between Amedica Corporation and James A. Youssef, M.D. dated as of May 8, 2004. | |
| 10.33n** | Amendment to Development Agreement by and between Amedica Corporation and James A. Youssef, M.D. dated as of May 24, 2006. | |
| 10.34n** | Development Agreement by and between Amedica Corporation and Jean-Jacques Abitol, M.D. dated as of February 16, 2005. | |
| 10.35n** | Development Agreement by and between Amedica Corporation and Gregg S. Gurwitz, M.D. dated as of February 21, 2005. | |
| 10.36n** | Development Agreement by and between Amedica Corporation and Andrew T. Dailey, M.D. dated as of April 19, 2005. | |
| 10.37n** | Development Agreement by and between Amedica Corporation and Scott D. Boden, M.D. dated as of July 21, 2005. | |
| 10.38n** | Development Agreement by and between Amedica Corporation and Carl Lauryssen, M.D. dated as of August 8, 2005. | |
| 10.39n** | Development Agreement by and between Amedica Corporation and Alan S. Hilibrand, M.D. dated as of September 8, 2005. | |
| 10.40n** | Development Agreement by and between Amedica Corporation and Rodney Plaster, M.D. dated as of December 12, 2005. | |
| 10.41n** | Development Agreement by and between Amedica Corporation and Michael P. Bolognesi, M.D. dated as of January 21, 2006. | |
| 10.42n** | Development Agreement by and between Amedica Corporation and Steven T. Lyons, M.D. dated as of February 20, 2006. | |
| 10.43n** | Development Agreement by and between Amedica Corporation and Harvinder S. Sandhu, M.D. dated as of May 31, 2006. | |
| 10.44n** | Development Agreement by and between Amedica Corporation and B. Sonny Bal, M.D. dated as of December 29, 2006. | |
II-5
| Exhibit Number |
Description of Exhibit | |
| 10.45n** | Amended and Restated Consulting Agreement by and between Amedica Corporation and Darrel S. Brodke, M.D. dated as of October 20, 2003. | |
| 10.46n** | Amendments to Amended and Restated Consulting Agreement by and between Amedica Corporation and Darrel S. Brodke, M.D. dated as of May 30, 2006. | |
| 10.50†** | 2007 Employee, Director And Consultant Equity Incentive Plan. | |
| 10.51†** | Form of 2007 Stock Option Agreement. | |
| 10.52†** | Form of 2007 Restricted Stock Agreement. | |
| 10.53** | Form of Indemnification Agreement by and between Amedica Corporation and its Officers and Directors. | |
| 23.1** | Consent of Ernst & Young LLP. | |
| 23.2 | Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (see Exhibit 5.1). | |
| 23.3** | Consent of LECG, LLC. | |
| 24.1** | Power of Attorney. | |
| * | To be filed by amendment. |
| ** | Previously filed. |
| n | Portions of this Exhibit have been omitted and filed separately with the Securities and Exchange Commission as part of an application for confidential treatment pursuant to the Securities Act of 1933, as amended. |
| † | Management contract or compensatory plan or arrangement. |
(b) Financial Statement Schedules
Financial Statement Schedules are omitted because the information is included in our financial statements or notes to those financial statements.
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described under Item 14 above, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-6
For the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date that it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of a registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or a prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
For the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering, containing material information about the undersigned registrant or its securities, provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
II-7
SIGNATURES AND POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this Amendment No. 4 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Los Angeles, California, on July 31, 2007.
| AMEDICA CORPORATION | ||
| By: |
/s/ Ashok C. Khandkar, Ph.D. | |
| Ashok C. Khandkar, Ph.D. Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 4 to the Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Ashok C. Khandkar, Ph.D. Ashok C. Khandkar, Ph.D. |
Chief Executive Officer and Director (principal executive officer) |
July 31, 2007 | ||
| * Warionex Belen |
President |
July 31, 2007 | ||
| /s/ Reyn E. Gallacher Reyn E. Gallacher |
Chief Financial Officer and Vice President, Finance (principal financial and accounting officer) |
July 31, 2007 | ||
| * Max Link, Ph.D. |
Chairman of the Board of Directors |
July 31, 2007 | ||
| * Aaron A. Hofmann, M.D. |
Director |
July 31, 2007 | ||
| * Lawrence D. Dorr, M.D. |
Director |
July 31, 2007 | ||
| * Gregg R. Honigblum |
Director |
July 31, 2007 | ||
| * Rohit Patel |
Director |
July 31, 2007 | ||
| * Bradford S. Goodwin |
Director |
July 31, 2007 | ||
By the signature set forth below, the undersigned, pursuant to the duly authorized powers of attorney filed with the Securities and Exchange Commission, has signed this Amendment No. 4 to the Registration Statement on behalf of the person indicated.
| * By: /s/ Ashok C. Khandkar, Ph.D. Ashok C. Khandkar, Ph.D. |
||||
II-8
Exhibit Index
| Number | Description of Exhibit | |
| 1.1** | Form of Underwriting Agreement. | |
| 3.1** | Amended and Restated Certificate of Incorporation of Amedica Corporation, as amended. | |
| 3.2** | Certificate of Amendment to Restated Certificate of Incorporation of Amedica Corporation. | |
| 3.3** |
Amended and Restated Certificate of Incorporation of Amedica Corporation to be effective upon completion of this offering. | |
| 3.4** |
Amended and Restated By-Laws of Amedica Corporation. | |
| 3.5** |
Amended and Restated By-Laws of Amedica Corporation to be effective upon completion of this offering. | |
| 4.1** |
Form of Common Stock Certificate. | |
| 4.2** | Third Amended and Restated Registration Rights Agreement by and among Amedica Corporation and certain securityholders of Amedica Corporation dated as of May 15, 2007. | |
| 4.3** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of March 1, 2004. | |
| 4.4** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as October 25, 2004. | |
| 4.5** | Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of February 24, 2006. | |
| 4.6** | Series D Warrant Agreement by and between Creation Capital LLC and Amedica Corporation dated as of April 27, 2007. | |
| 5.1 | Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., counsel to Amedica Corporation, with respect to the legality of securities being registered. | |
| 10.1** | Form of Subscription Agreement for Series C Convertible Preferred Stock. | |
| 10.2** | Form of Subscription Agreement for Series D Convertible Preferred Stock. | |
| 10.3†** |
Amedica Corporation 2003 Stock Option Plan. | |
| 10.4†** |
Form of 2003 Incentive Stock Option Agreement. | |
| 10.5†** |
Form of 2003 Non-Qualified Stock Option Agreement. | |
| 10.6†** |
Employment Offer Letter by and between Amedica Corporation and Eugene B. Jones dated April 2, 2004. | |
| 10.7†** |
Employment Offer Letter by and between Amedica Corporation and Bryan J. McEntire dated May 29, 2004. | |
| 10.8†** |
Employment Offer Letter by and between Amedica Corporation and Robert M. Wolfarth dated November 11, 2004. | |
| 10.9†** |
Employment Offer Letter by and between Amedica Corporation and Cameron G. Rouns dated December 17, 2004. | |
| 10.10†** |
Employment Offer Letter by and between Amedica Corporation and Warionex (“Jose”) Belen dated May 31, 2005. | |
| 10.11†** |
Employment Offer Letter by and between Amedica Corporation and Reyn E. Gallacher dated January 3, 2006. | |
| 10.12†** |
Employment Offer Letter by and between Amedica Corporation and Kenneth W. Ludwig dated May 10, 2006. | |
| 10.13†** |
Severance Agreement by and between Amedica Corporation and Ashok C. Khandkar dated May 23, 2005. | |
| 10.14†** |
Severance Agreement by and between Amedica Corporation and Bryan J. McEntire dated May 23, 2005. | |
| Number | Description of Exhibit | |
| 10.15†** |
Severance Agreement by and between Amedica Corporation and Warionex (“Jose”) Belen dated as of February 14, 2006. | |
| 10.16†** |
Severance Agreement by and between Amedica Corporation and Reyn E. Gallacher dated March 27, 2007. | |
| 10.17†** |
Severance Agreement by and between Amedica Corporation and Kenneth W. Ludwig dated March 27, 2007. | |
| 10.18†** |
Separation Agreement by and between Amedica Corporation and Eugene B. Jones dated January 5, 2007. | |
| 10.19** |
Assignment Agreement by and between Joint Enterprises, L.C. and Amedica Corporation dated August 1, 2001. | |
| 10.20** |
Amendment to Assignment by and between Joint Enterprises, L.C. and Amedica Corporation dated August 12, 2005. | |
| 10.21** |
Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of March 22, 2004. | |
| 10.22** |
First Amendment to Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of May 11, 2005. | |
| 10.23** |
Second Amendment to Lease by and between Paradigm Resources, L.C. and Amedica Corporation dated as of August 15, 2006. | |
| 10.24n** |
Letter Agreement between Creation Capital LLC and Amedica Corporation dated November 14, 2005. | |
| 10.25n** | Letter Agreement between Creation Capital LLC and Amedica Corporation dated March 26, 2007. | |
| 10.26n** |
Master Lease Agreement by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of April 28, 2006. | |
| 10.26.1** | Amendment of Master Lease by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of May 23, 2007. | |
| 10.27** |
Continuing Pledge Agreement by and between Chase Equipment Leasing, Inc. dated as of April 28, 2006. | |
| 10.27.1** | Security Agreement by and between Chase Equipment Leasing, Inc. and Amedica Corporation dated as of June 30, 2006. | |
| 10.28** |
Industrial Building Lease between 560 Arapeen LLC, Seventh Avenue LLC, First Avenue LLC, Alaska Limited Liability Companies and Amedica Corporation dated as of February 20, 2006. | |
| 10.29n** |
Product Development and License Agreement by and between Amedica Corporation and Dytech Corporation Ltd. dated December 20, 2006. | |
| 10.30n** |
Development Agreement by and between Amedica Corporation and Jeffrey C. Wang, M.D. dated as of May 8, 2004. | |
| 10.31n** |
Amendments to Development Agreement by and between Amedica Corporation and Jeffrey C. Wang, M.D. dated as of June 2, 2006. | |
| 10.32n** |
Development Agreement by and between Amedica Corporation and James A. Youssef, M.D. dated as of May 8, 2004. | |
| 10.33n** |
Amendment to Development Agreement by and between Amedica Corporation and James A. Youssef, M.D. dated as of May 24, 2006. | |
| 10.34n** | Development Agreement by and between Amedica Corporation and Jean-Jacques Abitol, M.D. dated as of February 16, 2005. | |
| 10.35n** | Development Agreement by and between Amedica Corporation and Gregg S. Gurwitz, M.D. dated as of February 21, 2005. | |
| 10.36n** |
Development Agreement by and between Amedica Corporation and Andrew T. Dailey, M.D. dated as of April 19, 2005. | |
| Number | Description of Exhibit | |
| 10.37n** |
Development Agreement by and between Amedica Corporation and Scott D. Boden, M.D. dated as of July 21, 2005. | |
| 10.38n** |
Development Agreement by and between Amedica Corporation and Carl Lauryssen, M.D. dated as of August 8, 2005. | |
| 10.39n** |
Development Agreement by and between Amedica Corporation and Alan S. Hilibrand, M.D. dated as of September 8, 2005. | |
| 10.40n** |
Development Agreement by and between Amedica Corporation and Rodney Plaster, M.D. dated as of December 12, 2005. | |
| 10.41n** |
Development Agreement by and between Amedica Corporation and Michael Bolognesi, M.D. dated as of January 21, 2006. | |
| 10.42n** |
Development Agreement by and between Amedica Corporation and Steve Lyons, M.D. dated as of February 20, 2006. | |
| 10.43n** |
Development Agreement by and between Amedica Corporation and Harvinder S. Sandhu, M.D. dated as of May 31, 2006. | |
| 10.44n** |
Development Agreement by and between Amedica Corporation and B. Sonny Bal, M.D. dated as of December 29, 2006. | |
| 10.45n** |
Amended and Restated Consulting Agreement by and between Amedica Corporation and Darrel S. Brodke, M.D. dated as of October 20, 2003. | |
| 10.46n** |
Amendments to Amended and Restated Consulting Agreement by and between Amedica Corporation and Darrel S. Brodke, M.D. dated as of May 30, 2006. | |
| 10.50†** | 2007 Employee, Director And Consultant Equity Incentive Plan. | |
| 10.51†** | Form of 2007 Stock Option Agreement. | |
| 10.52†** | Form of 2007 Restricted Stock Agreement. | |
| 10.53** | Form of Indemnification Agreement by and between between Amedica Corporation and each of its Officers and Directors. | |
| 23.1** | Consent of Ernst & Young LLP. | |
| 23.2 | Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (see Exhibit 5.1). | |
| 23.3** | Consent of LECG, LLC. | |
| 24.1** | Power of Attorney. | |
| * | To be filed by amendment. |
| ** | Previously filed. |
| n | Portions of this Exhibit have been omitted and filed separately with the Securities and Exchange Commission as part of an application for confidential treatment pursuant to the Securities Act of 1933, as amended. |
| † | Management contract or compensatory plan or arrangement. |